Portal maintenance status: (May 2019)
|
The Minerals Portal

In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.
The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks.
The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases.
Some natural solid substances without a definite crystalline structure, such as opal or obsidian, are more properly called mineraloids. If a chemical compound occurs naturally with different crystal structures, each structure is considered a different mineral species. Thus, for example, quartz and stishovite are two different minerals consisting of the same compound, silicon dioxide. (Full article...)
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...)
Selected articles
-
Image 1

Corundum is a crystalline form of aluminium oxide (Al2O3) typically containing traces of iron, titanium, vanadium, and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange.
The name "corundum" is derived from the Tamil-Dravidian word kurundam (ruby-sapphire) (appearing in Sanskrit as kuruvinda).
Because of corundum's hardness (pure corundum is defined to have 9.0 on the Mohs scale), it can scratch almost all other minerals. It is commonly used as an abrasive on sandpaper and on large tools used in machining metals, plastics, and wood. Emery, a variety of corundum with no value as a gemstone, is commonly used as an abrasive. It is a black granular form of corundum, in which the mineral is intimately mixed with magnetite, hematite, or hercynite.
In addition to its hardness, corundum has a density of 4.02 g/cm3 (251 lb/cu ft), which is unusually high for a transparent mineral composed of the low-atomic mass elements aluminium and oxygen. (Full article...) -
Image 2A lustrous crystal of zircon perched on a tan matrix of calcite from the Gilgit District of Pakistan
Zircon (/ˈzɜːrkɒn, -kən/) is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is ZrSiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green.
The name derives from the Persian zargun, meaning "gold-hued". This word is changed into "jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from Zirkon, which is the German adaptation of this word. Yellow, orange, and red zircon is also known as "hyacinth", from the flower hyacinthus, whose name is of Ancient Greek origin. (Full article...) -
Image 3
Graphite (/ˈɡræfaɪt/) is a crystalline allotrope (form) of the element carbon. It consists of many stacked layers of graphene, typically in the excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3 million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), lubricants (5%), among others (17%). Under extremely high pressures and extremely high temperatures it converts to diamond. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. (Full article...) -
Image 4
Opal is a hydrated amorphous form of silica (SiO2·nH2O); its water content may range from 3% to 21% by weight, but is usually between 6% and 10%. Due to the amorphous (chemical)physical structure, it is classified as a mineraloid, unlike crystalline forms of silica, which are considered minerals. It is deposited at a relatively low temperature and may occur in the fissures of almost any kind of rock, being most commonly found with limonite, sandstone, rhyolite, marl, and basalt.
The name opal is believed to be derived from the Sanskrit word upala (उपल), which means 'jewel', and later the Greek derivative opállios (ὀπάλλιος).
There are two broad classes of opal: precious and common. Precious opal displays play-of-color (iridescence); common opal does not. Play-of-color is defined as "a pseudo chromatic optical effect resulting in flashes of colored light from certain minerals, as they are turned in white light." The internal structure of precious opal causes it to diffract light, resulting in play-of-color. Depending on the conditions in which it formed, opal may be transparent, translucent, or opaque, and the background color may be white, black, or nearly any color of the visual spectrum. Black opal is considered the rarest, while white, gray, and green opals are the most common. (Full article...) -
Image 5
Cinnabar (/ˈsɪnəˌbɑːr/; from Ancient Greek κιννάβαρι (kinnábari)), or cinnabarite (/ˌsɪnəˈbɑːraɪt/), also known as mercurblende is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments.
Cinnabar generally occurs as a vein-filling mineral associated with volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and it exhibits birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning.
Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since as early as the Yangshao culture, where it was used in coloring stoneware. In Roman times, cinnabar was highly valued as paint for walls, especially interiors, since it darkened when used outdoors due to exposure to sunlight.
Associated modern precautions for the use and handling of cinnabar arise from the toxicity of the mercury component, which was recognized as early as ancient Rome. (Full article...) -
Image 6
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.
Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks.
The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide. (Full article...) -
Image 7Galena with minor pyrite
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. (Full article...) -
Image 8

Turquoise is an opaque, blue-to-green mineral that is a hydrous phosphate of copper and aluminium, with the chemical formula CuAl6(PO4)4(OH)8·4H2O. It is rare and valuable in finer grades and has been prized as a gemstone for millennia due to its hue.
Like most other opaque gems, turquoise has been devalued by the introduction of treatments, imitations, and synthetics into the market. The robin egg blue or sky blue color of the Persian turquoise mined near the modern city of Nishapur, Iran, has been used as a guiding reference for evaluating turquoise quality. (Full article...) -
Image 9
The mineral pyrite (/ˈpaɪraɪt/ PY-ryte), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of fool's gold. The color has also led to the nicknames brass, brazzle, and brazil, primarily used to refer to pyrite found in coal.
The name pyrite is derived from the Greek πυρίτης λίθος (pyritēs lithos), 'stone or mineral which strikes fire', in turn from πῦρ (pŷr), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what is now called pyrite.
By Georgius Agricola's time, c. 1550, the term had become a generic term for all of the sulfide minerals. (Full article...) -
Image 10
A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals:- Primitive cubic (abbreviated cP and alternatively called simple cubic)
- Body-centered cubic (abbreviated cI or bcc)
- Face-centered cubic (abbreviated cF or fcc)
Note: the term fcc is often used in synonym for the cubic close-packed or ccp structure occurring in metals. However, fcc stands for a face-centered-cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed.
Each is subdivided into other variants listed below. Although the unit cells in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. (Full article...) -
Image 11Dolomite (white) on talc
Dolomite (/ˈdɒl.əˌmaɪt, ˈdoʊ.lə-/) is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally CaMg(CO3)2. The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite (see Dolomite (rock)). An alternative name sometimes used for the dolomitic rock type is dolostone. (Full article...) -
Image 12
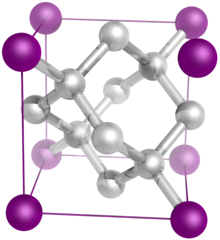
The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern.
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family.
The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (though there are many exceptions). (Full article...) -
Image 13

Zeolite exhibited in the Estonian Museum of Natural History
Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
1/n(AlO
2)−
(SiO
2)
x・yH
2O where Mn+
1/n is either a metal ion or H+.
The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone".
Zeolites occur naturally, but are also produced industrially on a large scale. As of December 2018[update], 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. (Full article...) -
Image 14The 423-carat (85 g) blue Logan Sapphire
Sapphire is a precious gemstone, a variety of the mineral corundum, consisting of aluminium oxide (α-Al2O3) with trace amounts of elements such as iron, titanium, cobalt, lead, chromium, vanadium, magnesium, boron, and silicon. The name sapphire is derived from the Latin word sapphirus, itself from the Greek word sappheiros (σάπφειρος), which referred to lapis lazuli. It is typically blue, but natural "fancy" sapphires also occur in yellow, purple, orange, and green colors; "parti sapphires" show two or more colors. Red corundum stones also occur, but are called rubies rather than sapphires. Pink-colored corundum may be classified either as ruby or sapphire depending on the locale. Commonly, natural sapphires are cut and polished into gemstones and worn in jewelry. They also may be created synthetically in laboratories for industrial or decorative purposes in large crystal boules. Because of the remarkable hardness of sapphires – 9 on the Mohs scale (the third-hardest mineral, after diamond at 10 and moissanite at 9.5) – sapphires are also used in some non-ornamental applications, such as infrared optical components, high-durability windows, wristwatch crystals and movement bearings, and very thin electronic wafers, which are used as the insulating substrates of special-purpose solid-state electronics such as integrated circuits and GaN-based blue LEDs. Sapphire is the birthstone for September and the gem of the 45th anniversary. A sapphire jubilee occurs after 65 years. (Full article...) -
Image 15

Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paints, and roofing material. It is a main ingredient in many cosmetics. It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak, though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc is not soluble in water, and is slightly soluble in dilute mineral acids.
Soapstone is a metamorphic rock composed predominantly of talc. (Full article...) -
Image 16

Micas (/ˈmaɪkəz/ MY-kəz) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites.
Micas are used in products such as drywalls, paints, and fillers, especially in parts for automobiles, roofing, and in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost". (Full article...) -
Image 17
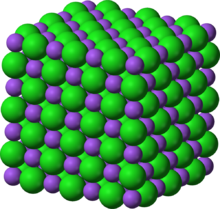
Crystal structure of table salt (sodium in purple, chlorine in green)
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
The smallest group of particles in material that constitutes this repeating pattern is unit cell of the structure. The unit cell completely reflects symmetry and structure of the entire crystal, which is built up by repetitive translation of unit cell along its principal axes. The translation vectors define the nodes of Bravais lattice.
The lengths of principal axes/edges, of unit cell and angles between them are lattice constants, also called lattice parameters or cell parameters. The symmetry properties of crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space may be described by 230 space groups.
The crystal structure and symmetry play a critical role in determining many physical properties, such as cleavage, electronic band structure, and optical transparency. (Full article...) -
Image 18A ruby crystal from Dodoma Region, Tanzania
Ruby is a pinkish red to blood-red colored gemstone, a variety of the mineral corundum (aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called sapphires. Ruby is one of the traditional cardinal gems, alongside amethyst, sapphire, emerald, and diamond. The word ruby comes from ruber, Latin for red. The color of a ruby is due to the element chromium.
Some gemstones that are popularly or historically called rubies, such as the Black Prince's Ruby in the British Imperial State Crown, are actually spinels. These were once known as "Balas rubies".
The quality of a ruby is determined by its color, cut, and clarity, which, along with carat weight, affect its value. The brightest and most valuable shade of red, called blood-red or pigeon blood, commands a large premium over other rubies of similar quality. After color follows clarity: similar to diamonds, a clear stone will command a premium, but a ruby without any needle-like rutile inclusions may indicate that the stone has been treated. Ruby is the traditional birthstone for July and is usually pinker than garnet, although some rhodolite garnets have a similar pinkish hue to most rubies. The world's most valuable ruby to be sold at auction is the Sunrise Ruby, which sold for US$34.8 million. (Full article...) -
Image 19

Green fluorite with prominent cleavage
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage". The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica".
Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral pattern with short covalent bonds. The planes of weakness (cleavage planes) in a diamond are in four directions, following the faces of the octahedron. In graphite, carbon atoms are contained in layers in a hexagonal pattern where the covalent bonds are shorter (and thus even stronger) than those of diamond. However, each layer is connected to the other with a longer and much weaker van der Waals bond. This gives graphite a single direction of cleavage, parallel to the basal pinacoid. So weak is this bond that it is broken with little force, giving graphite a slippery feel as layers shear apart. As a result, graphite makes an excellent dry lubricant.
While all single crystals will show some tendency to split along atomic planes in their crystal structure, if the differences between one direction or another are not large enough, the mineral will not display cleavage. Corundum, for example, displays no cleavage. (Full article...) -
Image 20
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond as a form of carbon is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high refractive index and a relatively high optical dispersion.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometres (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometres (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Natural and synthetic diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements. (Full article...) -
Image 21Amethyst cluster from Artigas, Uruguay
Amethyst is a violet variety of quartz. The name comes from the Koine Greek αμέθυστος amethystos from α- a-, "not" and μεθύσκω (Ancient Greek) methysko / μεθώ metho (Modern Greek), "intoxicate", a reference to the belief that the stone protected its owner from drunkenness. Ancient Greeks wore amethyst and carved drinking vessels from it in the belief that it would prevent intoxication.
Amethyst, a semiprecious stone, is often used in jewelry as gemstone bracelet, tumble, beads, cabochon etc. (Full article...) -
Image 22

Borax (also referred to as sodium borate, tincal (/ˈtɪŋkəl/) and tincar (/ˈtɪŋkər/)) is a salt (ionic compound), a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17.
It is a colorless crystalline solid that dissolves in water to make a basic solution.
It is commonly available in powder or granular form and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, and as a pharmaceutic alkalizer. In chemical laboratories, it is used as a buffering agent.
The terms tincal and tincar refer to native borax, historically mined from dry lake beds in various parts of Asia. (Full article...) -
Image 23

Kaolinite (/ˈkeɪ.ələˌnaɪt, -lɪ-/ KAY-ə-lə-nyte, -lih-; also called kaolin) is a clay mineral, with the chemical composition Al2Si2O5(OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica (SiO4) linked through oxygen atoms to one octahedral sheet of alumina (AlO6).
Kaolinite is a soft, earthy, usually white, mineral (dioctahedral phyllosilicate clay), produced by the chemical weathering of aluminium silicate minerals like feldspar. It has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g).
Rocks that are rich in kaolinite, and halloysite, are known as kaolin (/ˈkeɪ.əlɪn/) or china clay. In many parts of the world kaolin is colored pink-orange-red by iron oxide, giving it a distinct rust hue. Lower concentrations of iron oxide yield the white, yellow, or light orange colors of kaolin. Alternating lighter and darker layers are sometimes found, as at Providence Canyon State Park in Georgia, United States.
Kaolin is an important raw material in many industries and applications. Commercial grades of kaolin are supplied and transported as powder, lumps, semi-dried noodle or slurry. Global production of kaolin in 2021 was estimated to be 45 million tonnes, with a total market value of US $4.24 billion. (Full article...) -
Image 24

Garnets ( /ˈɡɑːrnɪt/) are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range [Mg,Fe,Mn]3Al2(SiO4)3; and uvarovite-grossular-andradite (ugrandite), with the composition range Ca3[Cr,Al,Fe]2(SiO4)3. (Full article...) -
Image 25

Tourmaline (/ˈtʊərməlɪn, -ˌliːn/ TOOR-mə-lin, -leen) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a wide variety of colors.
The name is derived from the Sinhalese tōramalli (ටෝරමල්ලි), which refers to the carnelian gemstones. (Full article...)
Selected mineralogist
-
Image 1Bernard (Bernie) Wood FRS MAE is a British geologist, and professor of mineralogy and senior research fellow at the University of Oxford. He specializes in the thermodynamics of geological systems, using experimental techniques. He is a prominent figure in the field of experimental petrology, having received multiple awards throughout his career and taught at several universities worldwide. (Full article...)
-
Image 2
Marcel Alexandre Bertrand (2 July 1847 – 13 February 1907) was a French geologist born in Paris. He was the son of mathematician Joseph Louis François Bertrand (1822–1900), and son-in-law to physicist Éleuthère Mascart (1837-1908).
He studied at the École Polytechnique, and beginning in 1869 he attended the Ecole des Mines de Paris. From 1877 he carried out geological mapping studies of Provence, the Jura Mountains and the Alps. In 1886, he became an instructor at the École Nationale Supérieure des Mines, and in 1896 became a member of the Académie des sciences. (Full article...) -
Image 3

Vasily Mikhailovich Severgin (Russian: Василий Михайлович Севергин; 19 September 1765 – 29 November 1826) was a Russian academician, chemist, mineralogist, and geologist. For three decades, he was the only academician elected to the Geological Society of London. He has been described as being among the most influential pioneers of geology in Russia. (Full article...) -
Image 4

Ernst Friedrich Germar
Ernst Friedrich Germar (3 November 1786 – 8 July 1853) was a German professor and director of the Mineralogical Museum at Halle. As well as being a mineralogist he was interested in entomology and particularly in the Coleoptera and Hemiptera. He wrote monographs on several insect families including the Scutelleridae. He also took an interest in paleoentomology. (Full article...) -
Image 5For the French Napoleonic War General see Marshall Étienne Macdonald
Marshall McDonald (October 18, 1835 – September 1, 1895) was an American engineer, geologist, mineralogist, pisciculturist, and fisheries scientist. McDonald served as the commissioner of the United States Commission of Fish and Fisheries from 1888 until his death in 1895. He is best known for his inventions of a number of fish hatching apparatuses and a fish ladder that enabled salmon and other migrating fish species to ascend the rapids of watercourses resulting in an increased spawning ground. McDonald's administration of the U.S. Commission of Fish and Fisheries was notably free of scandal and furthered the "protection and culture" of fish species throughout the United States. (Full article...) -
Image 6
Henri Longchambon (French pronunciation: [ɑ̃ʁi lɔ̃ʃɑ̃bɔ̃]; 27 July 1896 in Clermont-Ferrand, Puy-de-Dôme – 20 March 1969 in Le Kremlin-Bicêtre) was a French politician and scientist. (Full article...) -
Image 7

Robert Miller Hazen (born November 1, 1948) is an American mineralogist and astrobiologist. He is a research scientist at the Carnegie Institution of Washington's Geophysical Laboratory and Clarence Robinson Professor of Earth Science at George Mason University, in the United States. Hazen is the Executive Director of the Deep Carbon Observatory. (Full article...) -
Image 8Dr. E-An Zen (任以安) was born in Peking, China, May 31, 1928, and came to the U.S. in 1946. He became a citizen in 1963. Since 1990 he was adjunct professor at the University of Maryland. He died on March 29, 2014, at the age of 85.
He has contributed articles to professional journals and is a fellow of the Geological Society of America (Councillor, 1985–88, 1990–93; President, 1991–92); the American Association for the Advancement of Science (AAAS), the American Academy of Arts and Sciences, the Mineralogical Society of America (Council, 1974–77;Pres., 1975–76). He is a member of the Geological Society of Washington (Pres. 1973), the National Academy of Sciences, and the Mineralogical Association of Canada. Zen has been active in programs to bring geological knowledge to the general public. (Full article...) -
Image 9Carl Adolf Ferdinand Hintze (17 August 1851, Breslau – 28 December 1916, Breslau) was a German mineralogist and crystallographer.
From 1868 he studied at the University of Breslau, where he was a student of Ferdinand von Roemer. He then furthered his education at the universities of Bonn and Berlin. Beginning in 1872 he served as an assistant to mineralogist Paul Heinrich von Groth at the University of Strasbourg. In 1875, eye problems along with financial issues forced him to abandon his scientific activity at the university, and he subsequently found employment as a trader in the minerals business. Since 1880 he worked as a scientific director for a private firm in Bonn. (Full article...) -
Image 10

Carl Johann Bernhard Karsten
Karl Johann Bernhard Karsten (26 November 1782 – 22 August 1853) was a German mineralogist known for contributions made to the German metallurgy industry. (Full article...) -
Image 11
Jean-Étienne Guettard (22 September 1715 – 7 January 1786), was a French naturalist and mineralogist. He was born at Étampes, near Paris.
In boyhood, he gained a knowledge of plants from his grandfather, who was an apothecary, and later he qualified as a doctor in medicine. Pursuing the study of botany in various parts of France and other countries, he began to take notice of the relation between the distribution of plants and the soils and subsoils. In this way his attention came to be directed to minerals and rocks. (Full article...) -
Image 12Goethe in 1828, by Joseph Karl Stieler
Johann Wolfgang von Goethe (28 August 1749 – 22 March 1832) was a German polymath, who is widely regarded as the greatest and most influential writer in the German language. His work has had a profound and wide-ranging influence on Western literary, political, and philosophical thought from the late 18th century to the present day. A poet, playwright, novelist, scientist, statesman, theatre director, and critic, his works include plays, poetry and aesthetic criticism, as well as treatises on botany, anatomy, and color.
Goethe took up residence in Weimar in November 1775 following the success of his first novel, The Sorrows of Young Werther (1774), and joined a thriving intellectual and cultural environment under the patronage of Duchess Anna Amalia that had already included Abel Seyler's theatre company and Christoph Martin Wieland, and that formed the basis of Weimar Classicism. He was ennobled by the Duke of Saxe-Weimar, Karl August, in 1782. Goethe was an early participant in the Sturm und Drang literary movement. During his first ten years in Weimar, Goethe became a member of the Duke's privy council (1776–1785), sat on the war and highway commissions, oversaw the reopening of silver mines in nearby Ilmenau, and implemented a series of administrative reforms at the University of Jena. He also contributed to the planning of Weimar's botanical park and the rebuilding of its Ducal Palace. (Full article...) -
Image 13Peggy-Kay Hamilton (1922–1959) was born in Illinois in 1922 and was an American Research Associate in Mineralogy in the Department of Geology at Columbia University. One of Hamilton's first research breakthroughs was developing Research Project 49, otherwise known as the study of clay minerals. In her later research years, her focus shifted and led to her becoming involved full time in the study of uranium.
Hamilton achieved success in the fields of geology and mineralogy; according to her frequent research partner and friend Paul F. Kerr, Hamilton was held in high regard by both students at Columbia University as well as professional colleagues at multiple scientific research institutions. (Full article...) -
Image 14George "Shavey" Lorenzo Noyes (August 30, 1863 – 1945) was an American mineralogist, naturalist, development critic, writer and landscape artist. (Full article...)
-
Image 15
Alice Mary Dowse Weeks (August 26, 1909 – August 29, 1988) was an American geologist. Weeksite is named after her. She identified uranophane in 1953 along with Mary E. Thompson. Weeks was the first to propose the concept of oxidation of ore deposits that contain uranium, vanadium, and other accessory metals. She founded the Geology Department at Temple University in Philadelphia, and was a strong proponent of women in geology. (Full article...) -
Image 16

Sir H.Raeburn. Portrait of Sir G.S.Mackenzie,7th Bart. Size 63 x 41.5 in.
Sir George Steuart Mackenzie, 7th Baronet FRS FRSE FSA (22 June 1780–26 October 1848) was a Scottish geologist, chemist and agricultural improver. (Full article...) -
Image 17Clifford Howard Stockwell (September 26, 1897 – April 26, 1987) was a Canadian geologist, who published many scientific papers, reports and memoirs in the fields of Mineralogy, Structural Geology, Petrology, and Stratigraphy. He earned his PhD in geology at McGill University in Montreal in 1926. (Full article...)
-
Image 18George Thurland Prior FRS (16 December 1862 – 8 March 1936) was a British mineralogist. He made great contributions to mineralogical chemistry, petrology and meteoritics.
He was born in Oxford, England, and attended Magdalen College there in 1881. He received a first class in the Honour School in Chemistry in 1885 and Physics in 1886. Later he went to study in Germany. He obtained his Doctor of Science degree from Oxford University in 1905. (Full article...) -
Image 19

William Walter Jefferis (January 12, 1820 – February 23, 1906) was an American mineralogist and curator of the William S. Vaux Collection of minerals and artifacts at the Philadelphia Academy of Natural Sciences from 1883 to 1898. He personally collected and cataloged 35,000 mineral specimens, which he sold to the Carnegie Museum of Natural History in 1905. (Full article...) -
Image 20
Niels Steensen (Danish: Niels Steensen; Latinized to Nicolas Steno or Nicolaus Stenonius; 1 January 1638 – 25 November 1686 [NS: 11 January 1638 – 5 December 1686]) was a Danish scientist, a pioneer in both anatomy and geology who became a Catholic bishop in his later years. He has been beatified by the Catholic Church.
Steensen was trained in the classical texts on science; however, by 1659 he seriously questioned accepted knowledge of the natural world. Importantly he questioned explanations for tear production, the idea that fossils grew in the ground and explanations of rock formation. His investigations and his subsequent conclusions on fossils and rock formation have led scholars to consider him one of the founders of modern stratigraphy and modern geology. The importance of Steensen's foundational contributions to geology may be gauged from the fact that half of the twenty papers in a recent miscellany volume on The Revolution in Geology from the Renaissance to the Enlightenment focus on Steensen, the "preeminent Baroque polymath and founder of modern geologic thought". (Full article...) -
Image 21Hatten Schuyler Yoder, Jr., (March 20, 1921 – August 2, 2003) was a geophysicist and experimental petrologist who conducted pioneering work on minerals under high pressure and temperature. He was noted for his study of silicates and igneous rocks. (Full article...)
-
Image 22

René Just Haüy (French pronunciation: [aɥi]) FRS MWS FRSE (28 February 1743 – 1 June 1822) was a French priest and mineralogist, commonly styled the Abbé Haüy after he was made an honorary canon of Notre Dame. Due to his innovative work on crystal structure and his four-volume Traité de Minéralogie (1801), he is often referred to as the "Father of Modern Crystallography". During the French Revolution he also helped to establish the metric system. (Full article...) -
Image 23

André Laugier (1770–1832)
André Laugier (1 August 1770, in Lisieux – 19 April 1832, in Paris) was a French chemist, pharmacist and mineralogist. He was a cousin to famed chemist Antoine François Fourcroy and the father of astronomer Paul Auguste Ernest Laugier (1812–1872).
He received his education in his hometown of Lisieux, and during the French Revolution, was tasked with collecting church bells in Bretagne in order for them to be melted down for the production of cannons. In 1794 he was employed as head of the gunpowder and saltpeter works at the Comite de salut public. In 1797 he received his master's degree in pharmacy, and subsequently taught classes in chemistry and pharmacy at the military training schools in Toulon and Lille. (Full article...) -
Image 24Waldemar Theodore Schaller (August 3, 1882 – September 28, 1967) was an American mineralogist and longtime employee of the United States Geological Survey (USGS). (Full article...)
-
Image 25
Berend George Escher (4 April 1885 – 11 October 1967) was a Dutch geologist.
Escher had a broad interest, but his research was mainly on crystallography, mineralogy and volcanology. He was a pioneer in experimental geology. He was a half-brother of the artist M. C. Escher, and had some influence on his work due to his knowledge of crystallography. M.C. Escher created a woodcut ex libris for his brother 'Beer' with a stylized image of a volcano around 1922 (Bool number 91). (Full article...)
Related portals
Get involved
For editor resources and to collaborate with other editors on improving Wikipedia's Minerals-related articles, see WikiProject Rocks and minerals.
General images
-
Image 2Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)
-
Image 3Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral)
-
Image 6An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral)
-
Image 7Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral)
-
Image 8Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
-
Image 9Gypsum desert rose (from Mineral)
-
Image 10Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
-
Image 12Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
-
Image 14Mohs Scale versus Absolute Hardness (from Mineral)
-
Image 15Epidote often has a distinctive pistachio-green colour. (from Mineral)
-
Image 18Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral)
-
Image 19Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral)
-
Image 20When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
-
Image 21Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
-
Image 22Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral)
-
Image 24Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
-
Image 26Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
Did you know ...?
- ... that the mineral diaboleite (pictured) was so named out of desperation?
- ... that silicate perovskites may make up to 93% of the lower mantle and that the magnesium form is considered to be Earth's most abundant mineral?
- ... that while huemulite was discovered in 1959, it was not described until 1966?
Subcategories
Topics
| Overview | ||
|---|---|---|
| Common minerals | ||
Ore minerals, mineral mixtures and ore deposits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ores |
| ||||||||
| Deposit types | |||||||||
| Borates | |||||
|---|---|---|---|---|---|
| Carbonates | |||||
| Oxides |
| ||||
| Phosphates | |||||
| Silicates | |||||
| Sulfides | |||||
| Other |
| ||||
| Crystalline | |||||||
|---|---|---|---|---|---|---|---|
| Cryptocrystalline | |||||||
| Amorphous | |||||||
| Miscellaneous | |||||||
| Notable varieties |
| ||||||
| Oxide minerals |
| ||||
|---|---|---|---|---|---|
| Silicate minerals | |||||
| Other | |||||
Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
Mineral identification | |
|---|---|
| "Special cases" ("native elements and organic minerals") |
|
|---|---|
| "Sulfides and oxides" |
|
| "Evaporites and similars" |
|
| "Mineral structures with tetrahedral units" (sulfate anion, phosphate anion, silicon, etc.) |
|
Associated Wikimedia
The following Wikimedia Foundation sister projects provide more on this subject:
-
Commons
Free media repository -
Wikibooks
Free textbooks and manuals -
Wikidata
Free knowledge base -
Wikinews
Free-content news -
Wikiquote
Collection of quotations -
Wikisource
Free-content library -
Wikiversity
Free learning tools -
Wiktionary
Dictionary and thesaurus