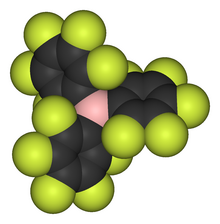
Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tris(pentafluorophenyl)borane | |
| Other names
Perfluorotriphenylboron
Tris(pentafluorophenyl)boron | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.101.316 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18F15B | |
| Molar mass | 511.98 g/mol |
| Appearance | colorless solid |
| Melting point | 126 to 131 °C (259 to 268 °F; 399 to 404 K) |
| forms adduct | |
| Structure | |
| trigonal planar | |
| 0 D | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Danger | |
| H315, H319, H335 | |
| P261, P280, P302+P352, P305+P351+P338 | |
| Related compounds | |
Related compounds
|
Triphenylborane (C6H5)3B BF3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editTris(pentafluorophenyl)borane is prepared using a Grignard reagent derived from bromopentafluorobenzene:
- 3C6F5MgBr + BCl3 → (C6F5)3B + 3MgBrCl
The synthesis originally employed C6F5Li, but this reagent can detonate with elimination of LiF.[2]
Structure
editThe structure of tris(pentafluorophenyl)borane (BCF) was determined by gas electron diffraction.[3] It has a propeller-like arrangement of its three pentafluorophenyl groups with a torsional angle of 40.6(3)° for the deviation of these groups from a hypothetically planar arrangement. Compared with a torsional angle of 56.8(4)° for tris(perfluoro-para-tolyl)borane, which is a stronger Lewis acid than BCF, this shows that there is some delocalization of electron density from the para-fluorine atoms to the boron atom that reduces its acidity.
Lewis acidity
editThe most noteworthy property of this molecule is its strong Lewis acidity. Its Lewis acid strength, as quantified by experimental equilibrium constants, is by 7 orders of magnitude higher than the one of structurally analogous triphenylborane.[4] Experimental equilibrium measurements, its AN value (Gutmann-Beckett method) as well as quantum-chemical calculations all indicate that the Lewis acidity of B(C6F5)3 is slightly lower than that of BF3 and significantly reduced compared to BCl3. B(C6F5)3 forms a strong Lewis adduct with water,[5] which was shown to be a strong Brønsted acid having an acidity comparable to hydrochloric acid (in acetonitrile).[6] In consequence, even traces of moisture are able to deactivate B(C6F5)3 and remaining catalytic activity might only be due to the Brønsted acidity of the water adduct.
Applications in catalysis
editIn one application (C6F5)3B forms noncoordinating anions by removing anionic ligands from metal centers.[7] Illustrative is a reaction that give rise to alkene polymerization catalysts where tris(pentafluorophenyl)boron is used as an activator or cocatalyst:
- (C6F5)3B + (C5H5)2Zr(CH3)2 → [(C5H5)2ZrCH3]+[(C6F5)3BCH3]−
In this process, the strongly coordinating methyl group transfers to the boron to expose a reactive site on zirconium. The resulting cationic zirconocene species is stabilised by the non coordinating borane anion. The exposed site on the zirconium allows for coordination of alkenes, whereupon migratory insertion into the remaining carbon-methyl ligand gives rise to a propyl ligand this process continues resulting in the growth of a polymer chain. This reagent has led to the development of immobilised catalyst/activator species; where the catalyst/activator is immobilised on an inert inorganic support such as silica.[8]
Tris(pentafluorophenyl)borane is also capable of abstracting hydride to give [(C6F5)3BH]−, and it catalyzes hydrosilylation of aldehydes. Otherwise (C6F5)3B binds to a wide range of Lewis bases, even weak ones.[9] The compound is hygroscopic, forming the trihydrate [(C6F5)3BOH2](H2O)2, wherein one water in coordinated to boron and the other two waters are hydrogen-bonded to the coordinated water.
Related compounds are pentafluorophenylboron halides.[10]
Frustrated Lewis pair
editTris(pentafluorophenyl)borane is a key reagent leading to the concept of frustrated Lewis pairs. The combination of BCF and bulky basic phosphines, such as tricyclohexylphosphine (PCy3) cleaves H2:[11]
- (C6F5)3B + PCy3 + H2 → (C6F5)3BH− + HPCy3+
Many related phosphines, boranes, and substrates participate in related reactions.
Other reactions
edit(C6F5)3B was used to prepare a compound containing a Xe-C bond:
- (C6F5)3B + XeF2 → [C6F5Xe]+[(C6F5)2BF2]−
Upon reaction with pentafluorophenyllithium, the salt of the noncoordinating anion lithium tetrakis(pentafluorophenyl)borate is formed.
- (C6F5)3B + C6F5Li → Li[(C6F5)4B]
B(C6F5)3 reacts with dimesitylphosphine to give the zwitterionic phosphonic-boronate (mes = C6H2Me3):
- (C6F5)3B + mes2PH → (C6F5)2B(F)−C6F4−P(H)mes2
This zwitterionic salt can be converted to a system that reversibly binds molecular H2:
- (C6F5)2B(F)−C6F4−P(H)mes2 + Me2SiHCl → (C6F5)2B(H)−C6F4−P(H)mes2 + Me2SiFCl
- (C6F5)2B(H)−C6F4−P(H)mes2 → (C6F5)2B−C6F4−Pmes2 + H2
References
edit- ^ GHS: Alfa Aesar L18054 (07 Jan 2021)
- ^ a b Piers, Warren E.; Chivers, Tristram (1997). "Pentafluorophenylboranes: from obscurity to applications". Chemical Society Reviews. 26 (5): 345. doi:10.1039/cs9972600345.
- ^ Körte, Leif A.; Schwabedissen, Jan; Soffner, Marcel; Blomeyer, Sebastian; Reuter, Christian G.; Vishnevskiy, Yury V.; Neumann, Beate; Stammler, Hans-Georg; Mitzel, Norbert W. (2017-06-09). "Tris(perfluorotolyl)borane-A Boron Lewis Superacid". Angewandte Chemie International Edition. 56 (29): 8578–8582. doi:10.1002/anie.201704097. ISSN 1433-7851. PMID 28524451.
- ^ Mayer, Robert J.; Hampel, Nathalie; Ofial, Armin R. (2020). "Lewis Acidic Boranes, Lewis Bases, and Equilibrium Constants: A Reliable Scaffold for a Quantitative Lewis Acidity/Basicity Scale". Chemistry – A European Journal. 27 (12): 4070–4080. doi:10.1002/chem.202003916. PMC 7985883. PMID 33215760.
- ^ Beringhelli, Tiziana; Maggioni, Daniela; D’Alfonso, Giuseppe (2001). "1H and 19F NMR Investigation of the Reaction of B(C6F5)3 with Water in Toluene Solution". Organometallics. 20 (23): 4927–4938. doi:10.1021/om010610n.
- ^ Bergquist, Catherine; Bridgewater, Brian M.; Harlan, C. Jeff; Norton, Jack R.; Friesner, Richard A.; Parkin, Gerard (2000). "Aqua, Alcohol, and Acetonitrile Adducts of Tris(perfluorophenyl)borane: Evaluation of Brønsted Acidity and Ligand Lability with Experimental and Computational Methods". Journal of the American Chemical Society. 122 (43): 10581–10590. doi:10.1021/ja001915g.
- ^ Fuhrmann, H.; Brenner, S.; Arndt, P.; Kempe, R. “Octahedral Group 4 Metal Complexes That Contain Amine, Amido, and Aminopyridinato Ligands: Synthesis, Structure, and Application in α-Olefin Oligo- and Polymerization”, Inorganic Chemistry, 1996, 35, 6742-6745.doi:10.1021/ic960182r
- ^ Severn, J. R., Chadwick, J. C., Duchateau, R., Friederichs, N., "Bound but Not Gagged‚ Immobilizing Single-Site α-Olefin Polymerization Catalysts", Chemical Reviews 2005, volume 105, p. 4073. doi:10.1021/cr040670d
- ^ Erker, G. "Tris(pentafluorophenyl)borane: A Special Boron Lewis Acid for Special Reactions", Dalton Transactions, 2005, 1883-1890. doi:10.1039/B503688G
- ^ Chivers, T. “Pentafluorophenylboron halides: 40 years later”, Journal of Fluorine Chemistry, 2002, 115, 1-8. doi:10.1016/S0022-1139(02)00011-8
- ^ Stephan, D. W., ""Frustrated Lewis Pairs": A New Strategy to Small Molecule Activation and Hydrogenation Catalysis", Dalton Trans. 2009, 3129.doi:10.1039/B819621D
Extra reading
edit- Lawson, James R.; Melen, Rebecca L. (3 February 2017). "Tris(pentafluorophenyl)borane and Beyond: Modern Advances in Borylation Chemistry". Inorganic Chemistry. 56 (15): 8627–8643. doi:10.1021/acs.inorgchem.6b02911. PMID 28157303.