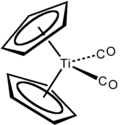
Dicarbonylbis(cyclopentadienyl)titanium is the chemical compound with the formula (η5-C5H5)2Ti(CO)2, abbreviated Cp2Ti(CO)2. This maroon-coloured, air-sensitive species is soluble in aliphatic and aromatic solvents.[1] It has been used for the deoxygenation of sulfoxides, reductive coupling of aromatic aldehydes and reduction of aldehydes.
| |
| |
| Names | |
|---|---|
| IUPAC name
dicarbonylbis(η5-cyclopentadienyl)titanium(II)
| |
| Other names
Dicarbonyldi-π-cyclopentadienyltitanium
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12H10O2Ti | |
| Molar mass | 234.09 g/mol |
| Appearance | maroon solid |
| Melting point | 90 °C (194 °F; 363 K) |
| Boiling point | Sublimes at 40 to 80 °C (104 to 176 °F; 313 to 353 K) at 0.001 mmHg |
| insoluble | |
| Solubility in other solvents | THF, benzene |
| Structure | |
| tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
flammable |
| Related compounds | |
Related compounds
|
Cp2TiCl2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and synthesis
editCp2Ti(CO)2 is prepared by the reduction of titanocene dichloride with magnesium as a slurry in THT under an atmosphere of carbon monoxide.[2]
- (C5H5)2TiCl2 + Mg + 2 CO → (C5H5)2Ti(CO)2 + MgCl2
Both Cp2Ti(CO)2 and Cp2TiCl2 are tetrahedral as are related zirconium and hafnium compounds. Of historical interest, the complex was first prepared by the reduction of titanocene dichloride with sodium cyclopentadienyl under an atmosphere of carbon monoxide.[3]
Its structure has been confirmed by X-ray crystallography.[4]
References
edit- ^ Sikora, D. J.; Moriarty, K. J.; Rausch, M. D. (1990). "Dicarbonylbis(η 5 -Cyclopentadienyl) Complexes of Titanium, Zirconium, and Hafnium". Inorganic Syntheses. Vol. 28. pp. 250–251. doi:10.1002/9780470132593.ch64. ISBN 978-0-471-52619-3.
- ^ Snead, Thomas E. (2001). "Dicarbonylbis(cyclopentadienyl)titanium". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289X.rd073. ISBN 0-471-93623-5.
- ^ Murray, James G. (1959). "A Metal Carbonyl Compound of Titanium". Journal of the American Chemical Society. 81 (3): 752–753. doi:10.1021/ja01512a062.
- ^ Atwood, Jerry L.; Stone, Karen E.; Alt, Helmut G.; Hrncir, Duane C.; Rausch, Marvin D. (1975). "Crystal and Molecular Structure of Titanocene Dicarbonyl, (η5-C5H5)2Ti(CO)2". Journal of Organometallic Chemistry. 96: C4–C6. doi:10.1016/S0022-328X(00)86431-1.