Transmembrane protein 8A is a protein that in humans is encoded by the TMEM8A gene (16p13.3.). Evolutionarily, TMEM8A orthologs are found in primates and mammals and in a few more distantly related species. TMEM8A contains five transmembrane domains and one EGF-like domain which are all highly conserved in the ortholog space. Although there is no confirmed function of TMEM8A, through analyzing expression and experimental data, it is predicted that TMEM8A is an adhesion protein that plays a role in keeping T-cells in their resting state.
| PGAP6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PGAP6, M83, TMEM6, TMEM8, transmembrane protein 8A, GPI-PLA2, TMEM8A, post-glycosylphosphatidylinositol attachment to proteins 6, post-GPI attachment to proteins 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 1926283; HomoloGene: 10944; GeneCards: PGAP6; OMA:PGAP6 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
editLocus
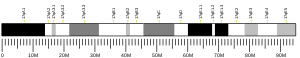
editThe human gene TMEM8A is found on chromosome 16 at the band 16p13.3.[4]
The span of this gene on chromosome 16 spans from base pair 420,773 to 437,113 making this gene 16,340 base pairs in length. This gene is found on the minus strand of the chromosome.[6] There are no known isoforms.
Aliases
editTMEM8A is also known as Transmembrane protein 8A, Transmembrane Protein 6, Five-Span Transmembrane Protein M83, TMEM6, TMEM8, Transmembrane protein 8 and M83.[7]
Homology
editParalogs
edit- There are two paralogs for TMEM8A found in humans, C9orf127 and TMEM8C. Both of these paralogs are found on Chromosome 9.[8]
Orthologs
edit- The ortholog space of TMEM8A is fairly narrow, with the majority of orthologs found in mammals and in particular primates with only a few exceptions.
| Genus and species[8] | Common name | Class | Accession | Percent identity |
|---|---|---|---|---|
| Pan troglodytes | Chimpanzee | Mammalia | XP_510709 | 99% |
| Pongo abelii | Orangutan | Mammalia | XP_002825960 | 97% |
| Macaca mulatta | Rhesus monkey | Mammalia | XP_001118461 | 93% |
| Callithrix jacchus | Marmoset | Mammalia | ABZ80344 | 93% |
| Rattus norvegicus | Rat | Mammalia | XP_001070176 | 43% |
| Bos taurus | Cow | Mammalia | NP_001092377 | 69% |
| Ailuropoda melanoleuca | Panda | Mammalia | EFB17134 | 78% |
| Canis familiaris | Dog | Mammalia | XP_003639163 | 74% |
| Equus caballus | Horse | Mammalia | XP_001915452 | 78% |
| Felis catus | Cat | Mammalia | XP_003999121 | 75% |
| Monodelphis domestica | Opossum | Mammalia | XP_001378110 | 44% |
| Ornithorhynchus anatinus | Platypus | Mammalia | XP_001512398 | 50% |
| Gallus gallus | Chicken | Aves | XP_003643243 | 56% |
| Anolis carolinensis | Lizard | Reptilia | XP_003230229 | 53% |
| Xenopus tropicalis | Frog | Amphibia | XP_002943473 | 43% |
| Takifugo rubripes | Pufferfish | Actinopterygii | XP_003975883 | 44% |
| Danio rerio | Zebrafish | Acrinoptergii | XP_00139040 | 44% |
| Strongylacentrotus putputatus | Sea urchin | Echinoidea | XP_795452 | 29% |
| Drosophila melanogaster | Fruit fly | Insecta | NP_651350 | 36% |
Protein
editPrimary sequence
editThe gene encodes a protein also called TMEM8A. This protein in 771 amino acids in length but has been shown to have a signal peptide from amino acid 1 to 34; the mature form of the protein is only 737 amino acids in length. The precursor form with signal peptide intact has a molecular weight of 84.780 kilodaltons and the mature form with the signal peptide cleaved has a molecular weight of 81.624 kilodaltons[9] TMEM8A has an isoelectric point of the mature form of pI=7.3.[10]
Domains and motifs
editTMEM8A is a transmembrane protein with five transmembrane domains, making it one of only three proteins found in the human body with five domains; the other two are CD47 and AC133. The protein also contains an EGF-like domain, which is a sequence of about thirty to forty amino-acid residues, found in the sequence of epidermal growth factor (EGF), that has been shown to be present in a more or less conserved form in a large number of other, mostly animal, proteins. The functional significance of EGF domains in what appear to be unrelated proteins is not yet clear. However, a common feature is that these repeats are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. The EGF domain includes six cysteine residues which have been shown (in EGF) to be involved in disulphide bonds. The main structure is a two-stranded beta-sheet followed by a loop to a short C-terminal two-stranded sheet. Subdomains between the conserved cysteines vary in length.[11]
Post-translational modifications
editThe protein has been shown to undergo glycosylation post-translationally at amino acids 144, 407, and 431.[12] There are also three disulfide bonds between amino acid 498 and 508, 502 and 521, and 523 and 532. These disulfide bonds are all characteristic of proteins with an EGF-like domain.
Secondary Structure
editExpression
editExpression
editTMEM8A is found to be expressed ubiquitously throughout the human body; however, it has been shown to be downregulated during CD4+ and CD8+ T-cell activation.[13]
Transcript variants
editThere are three natural transcript variants of TMEM8A. One is located at amino acid 136 where a threonine is swapped for an alanine. Another is present at amino acid 310 where an isoleucine is swapped for a valine and one at amino acid 567 where an arginine is swapped for a tryptophan. None of these variants result in a change of expression nor any loss/gain of function mutations.[14]
Interacting proteins
editTranscription factors
editThere are many predicted transcription factor binding sites in the TMEM8A promoter. Below is a table of the best possibilities, which have high confidence values, evolutionary conservation, and/or multiple possible binding sites in the promoter.
| Transcription Factor | Start | End | Strand | Sequence |
|---|---|---|---|---|
| Insulimoma associated factors | 1 | 13 | + | tggagGGGGtccg |
| Plemorphic adenoma gene 1 | 3 | 25 | + | gaGGGGgtccgggtggcagtgcg |
| Hypermethylated in cancer 1 | 11 | 23 | - | cacTGCCacccgg |
| Zinc finger with KRAB and SCAN domains 3 | 15 | 37 | - | gccatCCCCacccgcactgccac |
| Glial cells missing homolog 1 | 18 | 32 | + | ccccaCCCGcactgc |
| Basic krueppel-like factor (KLF3) | 19 | 35 | + | cagtgcGGGTggggatg |
| Zinc finger protein Spalt-2, sal-like 2, p150(sal2) | 23 | 33 | + | gcgggtGGGGatg |
| MYC-associated zinc finger protein related transcription factor | 23 | 35 | + | gcgggtGGGGatg |
| Myeloid zinc finger protein MZF1 | 27 | 37 | + | gtGGGGatggc |
| Hypermethylated in cancer 1 | 29 | 41 | - | gacTGCCatcccc |
| Yin and Yang 1 activator sites | 38 | 60 | - | gacactgCCATcgccactctgact |
| Y box binding protein 1, has a preference for binding ssDNA | 41 | 53 | + | cagagTGGCgatg |
| Glial cells missing homolog 1 | 54 | 68 | - | cgccaCCCGcactgc |
| Wilms Tumor Suppressor | 71 | 84 | + | ggcagtgTGGGtggcga |
| Runt-related transcription factor 2 / CBFA1 (core-binding factor, runt domain, alpha subunit 1) | 73 | 87 | + | cagtGTGGgtggcga |
| GLI-Kruppel family member GLI3 | 74 | 88 | - | atcgCCACccacact |
| Zinc finger with KRAB and SCAN domains 3 | 87 | 103 | - | gccatCCCCacccgcactgccat |
Interactions
editTMEM8A has been shown to interact with the following proteins
References
edit- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024180 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Martin J, Han C, Gordon LA, Terry A, Prabhakar S, She X, Xie G, Hellsten U, Chan YM, Altherr M, Couronne O, Aerts A, Bajorek E, Black S, Blumer H, Branscomb E, Brown NC, Bruno WJ, Buckingham JM, Callen DF, et al. (December 2004). "The sequence and analysis of duplication-rich human chromosome 16" (PDF). Nature. 432 (7020): 988–94. Bibcode:2004Natur.432..988M. doi:10.1038/nature03187. PMID 15616553. S2CID 4362044.
- ^ Dib C, Fauré S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996). "A comprehensive genetic map of the human genome based on 5,264 microsatellites". Nature. 380 (6570): 152–4. Bibcode:1996Natur.380..152D. doi:10.1038/380152a0. PMID 8600387. S2CID 28950185.
- ^ Daniels RJ, Peden JF, Lloyd C, Horsley SW, Clark K, Tufarelli C, Kearney L, Buckle VJ, Doggett NA, Flint J, Higgs DR (February 2001). "Sequence, structure and pathology of the fully annotated terminal 2 Mb of the short arm of human chromosome 16". Hum. Mol. Genet. 10 (4): 339–52. doi:10.1093/hmg/10.4.339. PMID 11157797.
- ^ "PGAP6 Gene - GeneCards | PGAP6 Protein | PGAP6 Antibody".
- ^ a b Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (October 1990). "Basic local alignment search tool". J. Mol. Biol. 215 (3): 403–10. doi:10.1016/S0022-2836(05)80360-2. PMID 2231712. S2CID 14441902.
- ^ "Tmem8 transmembrane protein 8 [Mus musculus (house mouse)] - Gene - NCBI".
- ^ Brendel V, Bucher P, Nourbakhsh IR, Blaisdell BE, Karlin S (March 1992). "Methods and algorithms for statistical analysis of protein sequences". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2002–6. Bibcode:1992PNAS...89.2002B. doi:10.1073/pnas.89.6.2002. PMC 48584. PMID 1549558.
- ^ Kansas GS, Saunders KB, Ley K, Zakrzewicz A, Gibson RM, Furie BC, Furie B, Tedder TF (February 1994). "A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion". J. Cell Biol. 124 (4): 609–18. doi:10.1083/jcb.124.4.609. PMC 2119911. PMID 7508943.
- ^ Prediction of N-glycosylation sites in human proteins. R. Gupta, E. Jung and S. Brunak. In preparation, 2004.
- ^ Motohashi T, Miyoshi S, Osawa M, Eyre HJ, Sutherland GR, Matsuda Y, Nakamura Y, Shibuya A, Iwama A, Nakauchi H (September 2000). "Molecular cloning and chromosomal mapping of a novel five-span transmembrane protein gene, M83". Biochem. Biophys. Res. Commun. 276 (1): 244–50. doi:10.1006/bbrc.2000.3409. hdl:2241/1570. PMID 11006113.
- ^ Ota T, Suzuki Y, Nishikawa T, et al. (January 2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.