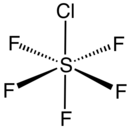
Sulfur chloride pentafluoride is an inorganic compound with the formula SF5Cl. It exists as a colorless gas at room temperature and is highly toxic, like most inorganic compounds containing the pentafluorosulfide (–SF5) functional group.[1] The compound adopts an octahedral geometry with C
4v symmetry. Sulfur chloride pentafluoride is the only commercially available reagent for adding the –SF5 group to organic compounds.[2][3]
| |||
| Names | |||
|---|---|---|---|
| Other names
Pentafluorochlorosulfanyl
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.034.014 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SF5Cl | |||
| Molar mass | 162.510 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 6.642 g/dm3 | ||
| Melting point | −64 °C (−83 °F; 209 K) | ||
| Boiling point | −19 °C (−2 °F; 254 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Reactivity
editSF5Cl is highly reactive and toxic. In contrast, sulfur hexafluoride (SF6) is inert and nontoxic despite having a closely related chemical formula. This difference highlights the lability of the S–Cl bond in SF5Cl.
Under free-radical conditions, SF5Cl adds across double bonds. The following reaction occurs with propene:
- CH
3CH=CH
2 + SF
5Cl → CH3CHClCH2SF5
The addition reaction is catalyzed by (CH3CH2)3B at around −30 °C. SF5Br is used similarly.[2]
SF5Cl is also a precursor to O(SF5)2 and F2NSF5 (from tetrafluorohydrazine).
Synthesis
editSulfur chloropentafluoride can be synthesized by several routes, starting from two lower sulfur fluorides, sulfur tetrafluoride and disulfur decafluoride:[1]
- SF4 + Cl2 + CsF → SF5Cl + CsCl
- ClF + SF4 → SF5Cl
- S2F10 + Cl2 → 2 SF5Cl
The corresponding SF
5Br is prepared similarly from in-situ generated bromine monofluoride.[4]
References
edit- ^ a b Nyman, F., Roberts, H. L., Seaton, T. "Sulfur Chloride Pentafluoride" Inorganic Syntheses, 1966, Volume 8, p. 160. doi:10.1002/9780470132395.ch42
- ^ a b Dolbier, William R.; et al. (2006). "A convenient and efficient method for incorporation of pentafluorosulfanyl (SF5) substituents into aliphatic compounds". Journal of Fluorine Chemistry. 127 (10): 1302–10. doi:10.1016/j.jfluchem.2006.05.003.
- ^ Savoie, Paul R.; Welch, John T. (2015). "Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds". Chemical Reviews. 115 (2): 1130–1190. doi:10.1021/cr500336u. PMID 25341449.
- ^ Winter, Rolf; Terjeson, Robin J.; Gard, Gary L. (1998). "An Improved and Facile Preparation of SF5Br". Journal of Fluorine Chemistry. 89: 105–106. doi:10.1016/S0022-1139(98)00094-3.