In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.
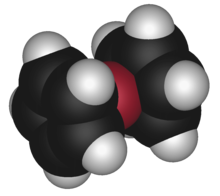
The term sandwich compound was introduced in organometallic nomenclature in 1956 in a report by J. D. Dunitz, L. E. Orgel and R. A. Rich, who confirmed the structure of ferrocene by X-ray crystallography.[1] The correct structure, in which the molecule features an iron atom sandwiched between two parallel cyclopentadienyl rings, had been proposed several years previously by Robert Burns Woodward and, separately, by Ernst Otto Fischer. The structure helped explain puzzles about ferrocene's conformers. This result further demonstrated the power of X-ray crystallography and accelerated the growth of organometallic chemistry.[2][page needed]
Classes
edit(Cycloheptatrienyl)(cyclopentadienyl)titanium (troticene) is an unsymmetrical sandwich complex.[3]
The best known members are the metallocenes of the formula M(C5H5)2 where M = Cr, Fe, Co, Ni, Pb, Zr, Ru, Rh, Os, Sm, Ti, V, Mo, W, Zn. These species are also called bis(cyclopentadienyl)metal complexes. Other arenes can serve as ligands as well.
- Mixed cyclopentadienyl complexes: M(C5H5)(CnHn). Some examples are Ti(C5H5)(C7H7) and (C60)Fe(C5H5Ph5) where the fullerene ligand is acting as a cyclopentadienyl analogue.
- Bis(benzene) complexes: M(C6H6)2, the best known example being bis(benzene)chromium.
- Bis(cyclooctatetraenyl) complexes: M(C8H8)2, such as U(C8H8)2 and Th(C8H8)2 (both actinocenes).
- Metal–carborane complexes (metallacarboranes), a very large and diverse family in which main-group or transition metal ions are coordinated to carborane ligands to form polyhedral cages ranging in size from 6 to 15 vertices. Examples include bis(dicarbollide) complexes,[4] such as [M(C2B9H11)2]z− and [Fe(C2B9H11)2]2−, and small-carborane sandwiches such as (R2C2B3H5)M(C2B4H6) and (R5C5)M(R′2)C2B4H4) where M is a transition metal and R and R′ are methyl or ethyl.[5][6]
Closely related are the metal complexes containing H3C3B2R2 (diborolyl) ligands.[7] In addition to these, other sandwich complexes containing purely inorganic ligands are known, such as Fe(C5Me5)(P5) and [(P5)2Ti]2−.[8]
Half-sandwich compounds
editHalf sandwich complexes have only one facially-bound planar organic ligand instead of two gives rise to a still larger family of half-sandwich compounds. One well studied example is probably methylcyclopentadienyl manganese tricarbonyl. Such species are occasionally referred to as piano-stool compounds, at least when there are three diatomic ligands. In such cases, the facially-bound planar organic ligand comprises the "seat" of the piano stool.
Multidecker sandwiches
editThe first isolated multidecker sandwich was the tris(cyclopentadienyl)dinickel triple-decker complex [Ni2Cp3]BF4, a highly air- and water-sensitive compound reported in 1972,[9] with X-ray crystallographic confirmation in 1974.[10]
In 1973 the electrically neutral air-stable triple-decker cobaltacarborane sandwiches 1,7,2,3- and 1,7,2,4-CpCo(RHC2B3H3)Cp (where R = H, Me) were isolated and characterized by multinuclear NMR and X-ray studies[11] (the structure of the 1,7,2,3 isomer is shown).
Since then many three-, four-, five-, and six-decker sandwich complexes have been described.[12][13] The largest structurally characterized multidecker sandwich monomer is the hexadecker shown at lower right.[14]
An extensive family of multidecker sandwiches incorporating planar (R2R′C3B2R″2)3− (diborolyl) ligands has also been prepared.[15]
Numerous multidecker sandwich compounds featuring hydrocarbon bridging rings have also been prepared, especially triple deckers.[16] A versatile method involves the attachment of Cp*Ru+ to preformed sandwich complexes.[17]
Linked sandwiches
editMonomeric double-decker and multidecker sandwiches have been used as building blocks for extended systems, some of which exhibit electron delocalization between metal centers. An example of a cyclic poly(metallacarborane) complex is the octahedral "carbon-wired" system shown below, which contains a planar C16B8 macrocycle.[18]
Inverse sandwiches
editIn these anti-bimetallic compounds, the metals are found to be bridged by a single carbocyclic ring. Examples include [(THF)3Ca]2(1,3,5-triphenylbenzene)[19] and [(Ar)Sn]2COT.
Double- and multimetallic sandwich compounds
editAnother family of sandwich compound involves more than one metal sandwiched between two carbocyclic rings. Examples of the double sandwich include V2(indenyl)2,[20] Ni2(COT)2[21] and Cr2(pentalene)2. Depicted at right is an example of a multimetallic sandwich compound, which has four palladium atoms joined in a chain sandwiched between two perylene units.[22] The counterions are bulky tetraarylborates.
Applications
editFerrocene and methylcyclopentadienyl manganese tricarbonyl have been used as antiknock agents. Certain bent metallocenes of zirconium and hafnium are effective precatalysts for the polymerization of propylene. Many half sandwich complexes of ruthenium, such as those derived from (cymene)ruthenium dichloride dimer catalyse transfer hydrogenation, a useful reaction in organic synthesis.[23][non-primary source needed]
Ferrocene derivatives have also been used as photoinitiators in cationic polymerization.[24]
References
edit- ^ Dunitz, J.; Orgel, L.; Rich, A. (1956). "The crystal structure of ferrocene". Acta Crystallographica. 9 (4): 373–375. doi:10.1107/S0365110X56001091.
- ^ Miessler, G. L.; Tarr, Donald A. (2004). Inorganic Chemistry. Upper Saddle River, NJ: Pearson Education. ISBN 0-13-035471-6.
- ^ Zeinstra, J.D.; De Boer, J.L. (1973). "Structure of Cyclopentadienylcycloheptatrienyl-titanium". Journal of Organometallic Chemistry. 54: 207–211. doi:10.1016/S0022-328X(00)85010-X.
- ^ a b Kang, H. C.; Lee, S. S.; Knobler, C. B.; Hawthorne, M. F. (1991). "Syntheses of Charge-Compensated Dicarbollide Ligand Precursors and Their Use in the Preparation of Novel Metallacarboranes". Inorganic Chemistry. 30 (9): 2024–2031. doi:10.1021/ic00009a015.
- ^ Grimes, R. N. (1999). "Small Carborane Ligands as Spectators and as Players". Journal of Organometallic Chemistry. 581 (1–2): 1–12. doi:10.1016/S0022-328X(99)00050-9.
- ^ Grimes, R. N. (2016). "13. Metallacarboranes of the Transition and Lanthanide Elements". Carboranes (3rd ed.). Oxford: Elsevier. ISBN 9780128019054.
- ^ Siebert, W. (1988). "Polydecker sandwich complexes". Pure and Applied Chemistry. 60 (8): 1345–1348. doi:10.1351/pac198860081345.
- ^ Urnezius, E.; Brennessel, W. W.; Cramer, C. J.; Ellis, J. E.; Schleyer, P. von R. (2002). "A Carbon-Free Sandwich Complex [(P5)2Ti]2−". Science. 295 (5556): 832–834. Bibcode:2002Sci...295..832U. doi:10.1126/science.1067325. PMID 11823635. S2CID 36455193.
- ^ Salzer, A.; Werner, H. (1972). "Studies on the Reactivity of Metal π‐Complexes. 6. A New Route to Triple‐Decker Sandwich Compounds". Angewandte Chemie International Edition. 11 (10): 930–932. doi:10.1002/anie.197209301.
- ^ Dubler, E.; Textor, M.; Oswald, H.-R.; Salzer, A. (1974). "X‐Ray Structure Analysis of the Triple‐Decker Sandwich Complex Tris(η‐cyclopentadienyl)dinickel Tetrafluoroborate". Angewandte Chemie International Edition. 13 (2): 135–136. doi:10.1002/anie.197401351.
- ^ a b Grimes, R. N.; Beer, D. C.; Sneddon, L. G.; Miller, V. R.; Weiss, R. (1974). "Small cobalt and nickel metallocarboranes from 2,3-dicarbahexaborane(8) and 1,6-dicarbahexaborane(6). Sandwich complexes of the cyclic C2B3H7(2^{-}) and C2B3H5(4^{-}) ligands". Inorganic Chemistry. 13 (5): 1138–1146. doi:10.1021/ic50135a025.
- ^ Grimes, R. N. (2007). "Boron-Containing Rings Ligated to Metals". In Crabtree, R. H.; Mingos, D. M. P. (eds.). Comprehensive Organometallic Chemistry III. Vol. 3. Oxford: Elsevier. pp. 1–48. doi:10.1016/B0-08-045047-4/00042-X. ISBN 978-0-08-045047-6.
- ^ Wang, X.; Sabat, M.; Grimes, R. N. (1995). "Organotransition-Metal Metallacarboranes. 43. Directed Synthesis of Carborane-End-Capped Multidecker Sandwiches". Journal of the American Chemical Society. 117 (49): 12218–12226. doi:10.1021/ja00154a023.
- ^ a b Wang, X.; Sabat, M.; Grimes, R. N. (1995). "Organotransition-Metal Metallacarboranes. 44. Construction of Pentadecker and Hexadecker Sandwiches from Triple-Decker Building Blocks". Journal of the American Chemical Society. 117 (49): 12227–12234. doi:10.1021/ja00154a024.
- ^ Siebert, W. (1993). "Di- and Trinuclear Metal Complexes of Diboraheterocycles". Advances in Organometallic Chemistry. 35: 187–210. doi:10.1016/S0065-3055(08)60491-8. ISBN 9780120311354.
- ^ Beck, V.; O'Hare, D. (2004). "Triple-decker transition metal complexes bridged by a single carbocyclic ring". Journal of Organometallic Chemistry. 689 (24): 3920–3938. doi:10.1016/j.jorganchem.2004.06.011.
- ^ Fagan, P. J.; Ward, M. D.; Calabrese, J. C. (1989). "Molecular engineering of solid-state materials: organometallic building blocks". Journal of the American Chemical Society. 111 (5): 1698–1719. doi:10.1021/ja00187a024.
- ^ Yao, H.; Sabat, M.; Grimes, R. N.; Fabrizi de Biani, F.; Zanello, P. (2003). "Organotransition‐Metal Metallacarboranes. 63. Metallacarborane‐Based Nanostructures: A Carbon‐Wired Planar Octagon". Angewandte Chemie International Edition. 42 (9): 1002–5. CiteSeerX 10.1.1.615.6577. doi:10.1002/anie.200390255. PMID 12616549.
- ^ Krieck, S.; Gorls, H.; Yu, L.; Reiher, M.; Westerhausen, M. (2009). "Stable "Inverse" Sandwich Complex with Unprecedented Organocalcium(I): Crystal Structures of [(thf)2Mg(Br)\sC6H2\s2,4,6\-Ph3] and [(thf)3Ca{μ\-C6H3\s1,3,5\-Ph3}Ca(thf)3]". Journal of the American Chemical Society. 131 (8): 2977–2985. doi:10.1021/ja808524y. PMID 19193100.
- ^ Jonas, K.; Rüsseler, W.; Krüger, C.; Raabe, E. (1986). "Synthesis of Diindenyldivanadium—a New Variant of the Reductive Degradation of Metallocenes and Related Compounds". Angewandte Chemie International Edition. 25 (10): 928–929. doi:10.1002/anie.198609281.
- ^ Brauer, D. J.; Kruger, C. (1976). "The stereochemistry of transition metal cyclooctatetraenyl complexes: di-η3,η3′-cyclooctatetraenedinickel, a sandwich compound with two enveloped nickel atoms". Journal of Organometallic Chemistry. 122: 265–273. doi:10.1016/S0022-328X(00)80619-1.
- ^ Murahashi, T.; Uemura, T.; Kurosawa, H. (2003). "Perylene–Tetrapalladium Sandwich Complexes". Journal of the American Chemical Society. 125 (28): 8436–8437. doi:10.1021/ja0358246. PMID 12848540.
- ^ Ikariya, T.; Hashiguchi, S.; Murata, K.; Noyori, R. (2005). "Preparation of Optically Active (R,R)-Hydrobenzoin from Benzoin or Benzil". Organic Syntheses. 82: 10. doi:10.15227/orgsyn.082.0010.
- ^ Dumur, F. (2020). Recent advances on iron-based photoinitiators of polymerization. European Polymer Journal, 139, 110026.