Protoparvovirus is a genus of viruses in the Parvovirinae subfamily of the virus family Parvoviridae.[2][3] Vertebrates serve as natural hosts. There are 15 species in the genus[4] including Rodent protoparvovirus 1 for which the exemplar virus is minute virus of mice (MVM). This genus also includes canine parvovirus (CPV), which causes gastrointestinal tract damage in puppies that is about 80% fatal,[5] and porcine parvovirus (PPV), which is a major cause of fetal death and infertility in pigs.[6] The genus divides phylogenetically into two branches, one that contains many founder members of the family, such as MVM, CPV and PPV, which have been studied in considerable detail, and a second branch occupied exclusively by predicted viruses whose coding sequences were identified recently in the wild using virus discovery approaches, but whose biology remains minimally explored. This second branch currently contains two species whose members infect humans, called Primate protoparvovirus 1 and Primate protoparvovirus 3. Until 2014, the genus was called Parvovirus, but it was renamed to eliminate confusion between members of this genus and members of the entire family Parvoviridae.[7][8]
| Protoparvovirus | |
|---|---|
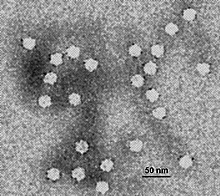
| |
| Electron micrograph of canine parvovirus | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Quintoviricetes |
| Order: | Piccovirales |
| Family: | Parvoviridae |
| Subfamily: | Parvovirinae |
| Genus: | Protoparvovirus |
| Species[1] | |
Taxonomy
edit15 species are currently recognized, many containing several named viruses, virus strains, genotypes or serotypes. When applied to viruses, the definition of species is a little unusual.[9] It is simply an abstract taxonomic concept that clusters a selected range of genetic variants, helping to distinguish branches in a phylogenetic lineage, but it is not a physical entity like a virus that can infect an animal or be isolated.[10] If the diversity level used to define a species is set very low, many will effectively contain a single virus, and the virus and species may even be given the same name, resulting in confusion between the two concepts in the literature, and marginalizing the phylogenetic role of the species taxon. To counter this problem, the diversity level now recognized for species in the Parvoviridae is relatively broad: species are defined as a cluster of similar viruses that encode a particular replication protein, typically called NS1, that is at least 85% identical to the protein encoded by other members of the species.[7][8]
Recognized species in genus Protoparvovirus include:[4]
- Carnivore protoparvovirus 1 (which includes the viruses canine parvovirus and feline parvovirus);
- Carnivore protoparvovirus 2
- Carnivore protoparvovirus 3
- Carnivore protoparvovirus 4
- Chiropteran protoparvovirus 1 (megabat bufavirus 1);
- Eulipotyphla protoparvovirus 1 (Mpulungu bufavirus);
- Primate protoparvovirus 1 (the human bufaviruses);
- Primate protoparvovirus 2 (the monkey virus Wuharv parvovirus 1);
- Primate protoparvovirus 3 (cutavirus);
- Primate protoparvovirus 4
- Rodent protoparvovirus 1 (which includes H-1 parvovirus, Kilham rat virus, LuIII virus, minute virus of mice, mouse parvovirus, tumor virus X, and rat minute virus);
- Rodent protoparvovirus 2 (rat parvovirus 1)
- Rodent protoparvovirus 3 (rat bufavirus SY-2015);
- Ungulate protoparvovirus 1 (porcine parvovirus)
- Ungulate protoparvovirus 2 (protoparvovirus Zsana/2013/HUN);
Protoparvoviruses that infect humans were first discovered in 2012 in the feces of children from Burkina Faso, and named using the siglum bufavirus.[11] Three genotypes of bufaviruses have so far been detected, circulating in Tunisia, Finland[12] and Bhutan[13]
A second virus in this genus that infects humans —cutavirus— was initially isolated from the feces of children with diarrhea.[14]
A third potential human protoparvovirus —tusavirus 1— has been reported in the feces of a single human, but whether or not it is able to infect humans or was simply ingested remains to be clarified.[15]
Structure
editViruses in genus Protoparvovirus have non-enveloped protein capsids around 18–26 nm in diameter, which show T=1 icosahedral symmetry. Genomes are single-stranded linear DNA between 4–6kb in length, with small (100–500b) imperfect palindromic sequences at each terminus that fold to form distinctive duplex hairpin telomeres.[5]
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Protoparvovirus | Icosahedral | T=1 | Non-enveloped | Linear ssDNA | No |
The capsid is thought to be made up of 60 VP proteins (PDB: 2CAS), both VP1 and VP2.
Genomic organization
editThe Protoparvovirus genome has two ORFs. The non-structural ORF (NS) is the "first" on the 5' side, with the structural (VP) on the 3' end. The genome is expressed by extensive alternative splicing. This not only allows for VP to be expressed, but also produces multiple alternatively spliced versions of each ORF, typically called NS1, NS2(P,Y,L), VP1, and VP2.[5] Some examples of annotated proteins from UniProt are:
- NS1 of the Minute virus of mice (MVM), P03134
- NS2 of MVM, P0DJZ2
- VP1 of canine parvovirus (CPV), P17455
- VP2 of CPV, P61826
Life cycle
editViral replication is nuclear. Entry into the host cell is achieved by attachment to host receptors, which mediates clathrin-mediated endocytosis. Replication follows the rolling-hairpin model. In some virus/host cell combinations, progeny virions can be trafficked through the cytoplasm in vesicles and released from the parental host cell prior to cell death, while the remaining virions are released following cell lysis. Vertebrates from 6 orders are currently known to serve as natural hosts. Transmission routes are typically fecal-oral and/or respiratory.[5]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Protoparvovirus | Vertebrates | Variable | endocytosis | early vesicular export/cell lysis | Nucleus | Nucleus | fecal-oral or respiratory |
History
editKilham rat virus, isolated in 1959,[16] was the first member of this family of small, linear, single-stranded DNA viruses to be identified.
In following years a series of physically similar viruses, including H1, LuIII, minute virus of mice and tumor virus X, were extracted from cells or tissues in routine use in research laboratories[17] and porcine parvovirus, PPV, one of the major causes of reproductive failure in swine, was isolated from infected pigs.[18] In 1971 these viruses were all recognized as part of a taxonomic genus called Parvovirus in the First Report of the newly created International Committee on Taxonomy of Viruses (ICTV)[19]
The Second ICTV Report, published in 1976, established the family Parvoviridae, which at that time included three genera, one of which retained the name Parvovirus and contained all of the aforementioned viruses plus feline panleukopenia virus (now called feline parvovirus, abbreviated to FPV), which had been shown to cause epidemics of enteritis, panleukopenia and congenital cerebellar ataxia in domestic cats. In 1978 a virus from the same species as FPV emerged that was able to infect dogs (called canine parvovirus or CPV), which rapidly spread globally, causing pandemics of severe intestinal and coronary disease.[20]
Genus Parvovirus continued to accrue new viruses until 2014, when its name was changed to Protoparvovirus.[7][8]
References
edit- ^ "Genus: Protoparvovirus" (html). International Committee on Taxonomy of Viruses (ICTV). Retrieved 8 January 2019.[dead link]
- ^ Cotmore, SF; Agbandje-McKenna, M; Canuti, M; Chiorini, JA; Eis-Hubinger, A; Hughes, J; Mietzsch, M; Modha, S; Ogliastro, M; Pénzes, JJ; Pintel, DJ; Qiu, J; Soderlund-Venermo, M; Tattersall, P; Tijssen, P; and the ICTV Report Consortium (2019). "ICTV Virus Taxonomy Profile: Parvoviridae". Journal of General Virology. 100 (3): 367–368. doi:10.1099/jgv.0.001212. PMC 6537627. PMID 30672729.
- ^ "ICTV 10th Report (2018) Parvoviridae".
- ^ a b "Virus Taxonomy: 2020 Release". International Committee on Taxonomy of Viruses (ICTV). March 2021. Retrieved 10 May 2021.
- ^ a b c d "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ^ "ICTV 10th Report (2018) Protoparvovirus".[dead link]
- ^ a b c "ICTV Official Taxonomy: Updates since the 8th Report". ICTV Official Taxonomy. Archived from the original on 19 May 2014. Retrieved 11 June 2014.
- ^ a b c Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Gatherer D, Davison AJ. 2014. The family Parvoviridae. Arch. Virol. 159: 1239–47.
- ^ Van Regenmortel MHV (2000) Introduction to the species concept in virus taxonomy. In: Van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, McGeogh DJ, Maniloff J, Mayo MA, Pringle CR, Wickner RB. (eds) Virus Taxonomy-Seventh Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London.
- ^ Van Regenmortel MHV (2003) Viruses are real, virus species are man-made taxonomic constructions. Arch. Virol. 148: 2481–2488.
- ^ Phan TG, Vo NP, Bonkoungou IJ, Kapoor A, Barro N, O’Ryan M, Kapusinszky B, Wang C, Delwart E. 2012. Acute diarrhea in West African children: diverse enteric viruses and a novel parvovirus genus. J Virol. 86:11024–30
- ^ Väisänen E, Kuisma I, Phan TG, Delwart E, Lappalainen M, Tarkka E, Hedman K, Söderlund-Venermo M. 2014. Bufavirus in feces of patients with gastroenteritis, Finland. Emerg Infect Dis. 20(6):1078–80.
- ^ Yahiro T, Wangchuk S, Tshering K, Bandhari P, Zangmo S, Dorji T, Tshering K, Matsumoto T, Nishizono A, Söderlund-Venermo M, Ahmed K. 2014. Novel human bufavirus genotype 3 in children with severe diarrhea, Bhutan. Emerg Infect Dis. 20(6):1037–9.
- ^ Phan TG, Dreno B, da Costa AC, Li L, Orlandi P, Deng X, Kapusinszky B, Siqueira J, Knol AC, Halary F, Dantal J, Alexander KA, Pesavento PA, Delwart E A new protoparvovirus in human fecal samples and cutaneous T cell lymphomas (mycosis fungoides). Virology 496:299–305. doi: 10.1016/j.virol.2016.06.013
- ^ Phan TG, Sdiri-Loulizi K, Aouni M, Ambert-Balay K, Pothier P, Deng X, Delwart E (2014) New parvovirus in child with unexplained diarrhea, Tunisia. Emerg Infect Dis 20(11):1911–1913. doi: 10.3201/eid2011.140428
- ^ Kilham, L. and Olivier, L. J. 1959. A latent virus of rats isolated in tissue culture. Virology 7: 428–37
- ^ Tattersall, P. 2006. The evolution of parvovirus taxonomy. In: ‘‘The Parvoviruses’’. J. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (eds), Chap. 1, pp. 5–4. Hodder Arnold, London.
- ^ Ren X, Tao Y, Cui J, Suo S, Cong Y, Tijssen P. 2013. Phylogeny and evolution of porcine parvovirus. Virus Research. 178:392–397.
- ^ "International Committee on Taxonomy of Viruses (ICTV)".
- ^ Hoelzer K, Parrish CR. 2010. The emergence of parvoviruses of carnivores. Vet. Res. 41:39.