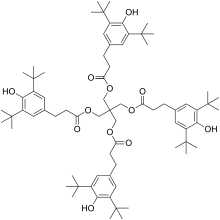
Pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate) is a chemical compound composed of four sterically hindered phenols linked through a pentaerythritol core. It is used as primary antioxidant for stabilizing polymers, particularly polyethylene and polypropylene.
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis({[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoyl]oxy}methyl)propane-1,3-diyl bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate] | |
| Other names
Anox 20, Irganox 1010, Dovernox 10
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.021 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C73H108O12 | |
| Molar mass | 1177.655 g·mol−1 |
| Appearance | White solid |
| Melting point | 110–125 °C (230–257 °F; 383–398 K) |
| <0.1 g/ml | |
| Solubility in Acetone | 0.75 g/ml |
| Solubility in Toluene | 0.5 g/ml |
| Solubility in Methanol | <0.1 g/ml |
| Hazards | |
| GHS labelling: | |
| H413 | |
| P273, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editBase catalysed Michael addition of methyl acrylate to 2,6-di-tert-butylphenol forms the intermediate butyl-phloretic ester. High temperature transesterification of this with pentaerythritol gives the final product. Driving this reaction to completion can be difficult and commercial samples often contain low levels of the tri-ester.
Properties
editThe linking of phenols together with pentaerythritol maintains their activity with greatly reduced volatility. This is important during the processing and molding steps where the plastic is heated to molten, typically several hundred degrees.[1]
See also
edit- Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate - an inexpensive and commonly used polymer stabiliser
- Tris(3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate - a polymer stabiliser commonly used to protect against long term heat aging
- Irganox 1098 - a polymer stabiliser with metal chelation properties
References
edit- ^ Vulic, Ivan; Vitarelli, Giacomo; Zenner, John M. (January 2002). "Structure–property relationships: phenolic antioxidants with high efficiency and low colour contribution". Polymer Degradation and Stability. 78 (1): 27–34. doi:10.1016/S0141-3910(02)00115-5.