The p24 capsid protein is the most abundant HIV protein with each virus containing approximately 1,500 to 3,000 p24 molecules.[1] It is the major structural protein within the capsid, and it is involved in maintaining the structural integrity of the virus and facilitating various stages of the viral life cycle, including viral entry into host cells and the release of new virus particles.[2] Detection of p24 protein's antigen can be used to identify the presence of HIV in a person's blood and diagnose HIV/AIDS, however, more modern tests have taken their place.[3] After approximately 50 days of infection, the p24 antigen is often cleared from the bloodstream entirely.[4]
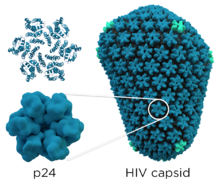
Structure
editP24 has a molecular weight of 24 kDa and is encoded by the gag gene. The structure of HIV capsid was determined by X-ray crystallography and cryo-electron microscopy.[5] The p24 capsid protein consists of two domains: the N-terminal domain and the C-terminal domain connected by flexible inter-domain linkers. The N-terminal domain (NTD) is made up of 7 α-helices (H) and β-hairpin.[6][7] The C-terminal domain (CTD) has 4 α-helices and an 11-residue unstructured region.[8][9] The N-terminal domain (NTD) facilitates contacts within the hexamer, while the C-terminal domain (CTD) forms dimers that bind to adjacent hexamers.[10] Each hexamer contains a size-selective pore surrounded by six positively charged arginine residues, and the pore is covered by a β-hairpin that can undergo conformational changes, which has both open and closed conformations.[11] At the center of the hexamers lies an IP6 molecule which stabilizes the tertiary structure of the molecule. Additionally, the C-terminal domain includes a major homology region (MHR) spanning amino acids 153 to 172 with 20 highly conserved amino acids.[11] Moreover, the N-terminal domain features a loop (amino acids 85–93) that interacts with the protein cyclophilin A (Cyp A).
Function
editP24 is a structural protein that plays a crucial role in the formation and stability of the viral capsid, which protects the viral RNA. p24 capsid protein's roles in the HIV replicative process are summarized as follows:[citation needed]
- Fusion: HIV replication cycle begins when HIV fuses with the surface of the host cell. The capsid containing the virus’s genome and proteins then enters the cells.
- Reverse transcription: The capsid ensures the secure transport of the viral genome and reverse-transcription machinery from the cytoplasm's periphery to transcriptionally active sites in the nucleus. It achieves this by shielding the viral genome from detection by restriction factors, while still allowing the necessary molecules to diffuse through the core, facilitating the process of reverse transcription.
- Assembly: It is involved in the assembly of new virus particles, facilitating the proper organization of viral components.
- Budding: P24 contributes to the viral budding process, ensuring the proper packaging and release of mature and infectious virus particles.
p24 HIV capsid as a therapeutic target
editNew antiretroviral therapy
editCyclosporine, an immunosuppressant drug designed to prevent organ transplant rejection, has been shown to inhibit infection in HIV-1 positive people.[12] Cyclosporine acts as a competitive inhibitor to the capsid protein’s association with CypA, a cellular protein. CypA has been shown to be important for HIV’s infectivity.
The HIV-1 p24 capsid protein plays crucial roles throughout the replication cycle, making it an attractive therapeutic target. Unlike the viral enzymes (protease, reverse transcriptase and integrase) that are currently targeted by small-molecule antiretroviral drugs, p24 capsid proteins operate through protein-protein interactions. Capsid inhibitors, such as Lenacapavir and GS-6207, interfere with the activities of the HIV capsid protein and underwent evaluation in phase-1 clinical trials as monotherapies.[13][14] They demonstrated anti-viral activity against all subtypes with no cross-resistance with current antiretroviral drugs.[13][14] These findings support therapies aimed at disrupting the functions of the HIV capsid protein.
Vaccine design
editP24 can induce cellular immune responses and has been included in some vaccine strategies.[3]
Diagnosis
editFourth generation-HIV test
editP24 is a target for the immune system, and antibodies against p24 are used in diagnostic tests to detect the presence of HIV antibodies. Fourth-generation HIV immunoassays detect viral p24 protein in the blood and patient antibodies against the virus. Previous generation tests relied on detecting patient antibodies alone; it takes about 3–4 weeks for the earliest antibodies to be detected. The p24 protein can be detected in a patient's blood as early as 2 weeks after infection, further reducing the window period necessary to accurately detect the HIV status of the patient.[15]
See also
editReferences
edit- ^ Summers MF, Henderson LE, Chance MR, Bess JW, South TL, Blake PR, et al. (May 1992). "Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1". Protein Science. 1 (5): 563–574. doi:10.1002/pro.5560010502. PMC 2142235. PMID 1304355.
- ^ Rossi E, Meuser ME, Cunanan CJ, Cocklin S (January 2021). "Structure, Function, and Interactions of the HIV-1 Capsid Protein". Life. 11 (2): 100. Bibcode:2021Life...11..100R. doi:10.3390/life11020100. PMC 7910843. PMID 33572761.
- ^ a b Larijani MS, Sadat SM, Bolhassani A, Pouriayevali MH, Bahramali G, Ramezani A (2019). "In Silico Design and Immunologic Evaluation of HIV-1 p24-Nef Fusion Protein to Approach a Therapeutic Vaccine Candidate". Current HIV Research. 16 (5): 322–337. doi:10.2174/1570162x17666190102151717. PMC 6446525. PMID 30605062.
- ^ Hurt CB, Nelson JA, Hightow-Weidman LB, Miller WC (December 2017). "Selecting an HIV Test: A Narrative Review for Clinicians and Researchers". Sexually Transmitted Diseases. 44 (12): 739–746. doi:10.1097/OLQ.0000000000000719. PMC 5718364. PMID 29140890.
- ^ Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, et al. (May 2013). "Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics". Nature. 497 (7451): 643–646. Bibcode:2013Natur.497..643Z. doi:10.1038/nature12162. PMC 3729984. PMID 23719463.
- ^ Gitti RK, Lee BM, Walker J, Summers MF, Yoo S, Sundquist WI (July 1996). "Structure of the amino-terminal core domain of the HIV-1 capsid protein". Science. 273 (5272): 231–235. Bibcode:1996Sci...273..231G. doi:10.1126/science.273.5272.231. PMID 8662505. S2CID 5960953.
- ^ Momany C, Kovari LC, Prongay AJ, Keller W, Gitti RK, Lee BM, et al. (September 1996). "Crystal structure of dimeric HIV-1 capsid protein". Nature Structural Biology. 3 (9): 763–770. doi:10.1038/nsb0996-763. PMID 8784350. S2CID 33672057.
- ^ Du S, Betts L, Yang R, Shi H, Concel J, Ahn J, et al. (February 2011). "Structure of the HIV-1 full-length capsid protein in a conformationally trapped unassembled state induced by small-molecule binding". Journal of Molecular Biology. 406 (3): 371–386. doi:10.1016/j.jmb.2010.11.027. PMC 3194004. PMID 21146540.
- ^ Gamble TR, Yoo S, Vajdos FF, von Schwedler UK, Worthylake DK, Wang H, et al. (October 1997). "Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein". Science. 278 (5339): 849–853. Bibcode:1997Sci...278..849G. doi:10.1126/science.278.5339.849. PMID 9346481.
- ^ Tan A, Pak AJ, Morado DR, Voth GA, Briggs JA (January 2021). "Immature HIV-1 assembles from Gag dimers leaving partial hexamers at lattice edges as potential substrates for proteolytic maturation". Proceedings of the National Academy of Sciences of the United States of America. 118 (3). Bibcode:2021PNAS..11820054T. doi:10.1073/pnas.2020054118. PMC 7826355. PMID 33397805.
- ^ a b Obr M, Kräusslich HG (July 2018). "The secrets of the stability of the HIV-1 capsid". eLife. 7: e38895. doi:10.7554/eLife.38895. PMC 6067877. PMID 30063007.
- ^ Sokolskaja, Elena; Olivari, Silvia; Zufferey, Madeleine; Strambio-De-Castillia, Caterina; Pizzato, Massimo; Luban, Jeremy (May 2010). "Cyclosporine Blocks Incorporation of HIV-1 Envelope Glycoprotein into Virions". Journal of Virology. 84 (9): 4851–4855. doi:10.1128/JVI.01699-09. ISSN 0022-538X. PMC 2863729. PMID 20181694.
- ^ a b Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, et al. (August 2020). "Clinical targeting of HIV capsid protein with a long-acting small molecule". Nature. 584 (7822): 614–618. Bibcode:2020Natur.584..614L. doi:10.1038/s41586-020-2443-1. PMC 8188729. PMID 32612233.
- ^ a b Dvory-Sobol H, Shaik N, Callebaut C, Rhee MS (January 2022). "Lenacapavir: a first-in-class HIV-1 capsid inhibitor". Current Opinion in HIV and AIDS. 17 (1): 15–21. doi:10.1097/COH.0000000000000713. PMID 34871187. S2CID 244940471.
- ^ Constantine N (February 1998). "HIV Antibody Assays". HIV InSite Knowledge Base. Archived from the original on 2001-06-25 – via hivinsite.ucsf.edu.
Further reading
edit- Wiznerowicz M. "TU vs pg of p24". Trono Lab – Laboratory of Virology and Genetics. École polytechnique fédérale de Lausanne (EPFL). Archived from the original on 9 March 2008.
- "Frequently Asked Questions: Fourth Generation HIV Ab/Ag Combination Assays" (PDF). PA/ MidAtlantic AIDS Education and Training Center at the Health Federation of Philadelphia Last Revised. March 2013. Archived from the original (PDF) on 15 July 2015.