The topic of this article may not meet Wikipedia's general notability guideline. (December 2017) |
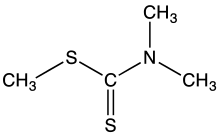
Methyl dimethyldithiocarbamate is the organosulfur compound with the formula (CH3)2NC(S)SCH3. It is the one of simplest dithiocarbamic esters. It is a white volatile solid that is poorly soluble in water but soluble in many organic solvents. It was once used as a pesticide.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl dimethylcarbamodithioate | |
| Other names
Cystogon, DMDTM, Forbiat
| |
| Identifiers | |
| ECHA InfoCard | 100.021.005 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C4H9NS2 | |
| Molar mass | 135.24 g·mol−1 |
| Appearance | colorless or white solid |
| Melting point | 45–47 °C (113–117 °F; 318–320 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl dimethyldithiocarbamate can be prepared by methylation of salts of dimethyldithiocarbamate:[1]
- (CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na[CH3OSO3]
It can also be prepared by the reaction of a tetramethylthiuram disulfide with methyl Grignard reagents:[2]
- [(CH3)2NC(S)S]2 + CH3MgBr → (CH3)2NC(S)SCH3 + (CH3)2NCS2MgBr
References
edit- ^ A. D. Ainley; W. H. Davies; H. Gudgeon; J. C. Harland; W. A. Sexton (1944). "The Constitution of the So-Called Carbothialdines and the Preparation of Some Homologous Compounds". J. Chem. Soc.: 147–152. doi:10.1039/JR9440000147.
- ^ John R. Grunwell (1970). "Reaction of Grignard Reagents with Tetramethylthiuram Disulfide [yielding dithiocarbamates]". J. Org. Chem. 35 (5): 1500–1501. doi:10.1021/jo00830a052.