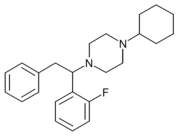
MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co.[1] It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer (the opposite stereochemistry from the related drug lefetamine).[2][3] It has been used as a lead compound from which a large family of potent opioid drugs[4] have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes.[5][6][7][8][9][10] Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.[11]
 | |
| Clinical data | |
|---|---|
| Other names | MT-45, IC-6 |
| Routes of administration | oral administration rectal administration |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H32N2 |
| Molar mass | 348.534 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Side effects
editRecreational use of MT-45 has been associated with unconsciousness and overdose, as well as a range of unusual side effects not typically seen with other opioid agonists, including hearing loss, hair depigmentation, alopecia, cataracts, and skin and nail reactions such as dermatitis and Mees lines. The cause for this is unclear, although a structural similarity to a withdrawn drug triparanol which caused similar side effects has been noted.[12][13][14][15][16][17]
Legality
editMT-45 became a class A drug in the UK on 11 March 2015.[18]
MT-45 is banned in the Czech Republic.[19]
The Canadian Controlled Drugs and Substances Act was amended in 2016 to include the substance as a Schedule I substance. Possession without legal authority can result in maximum 7 years imprisonment. Further, Health Canada amended the Food and Drug Regulations in May 2016 to classify MT-45 as a restricted drug.[20] Only those with a law enforcement agency, person with an exemption permit or institutions with Minister's authorization may possess the drug in Canada.
In the United States, the DEA placed MT-45 in Schedule 1 of the Controlled Substance Act. This took effect on January 12, 2018.[21]
See also
editReferences
edit- ^ US patent 3957788, Haruki Nishimura, Hitoshi Uno, Kagayaki Natsuka, Noriaki Shimokawa, Masanao Shimizu, Hideo Nakamura, "1-Substituted-4-(1,2-diphenylethyl)piperazine derivatives and their salts", published 1975-15-01, issued 1976-18-05
- ^ Natsuka K, Nakamura H, Uno H, Umemoto S (December 1975). "Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 1". Journal of Medicinal Chemistry. 18 (12): 1240–4. doi:10.1021/jm00246a014. PMID 1195277.
- ^ Nakamura H, Shimizu M (May 1976). "Comparative study of 1-cyclohexyl-4-(1,2-diphenylethyl)-piperazine and its enantiomorphs on analgesic and other pharmacological activities in experimental animals". Archives Internationales de Pharmacodynamie et de Thérapie. 221 (1): 105–21. PMID 962421.
- ^ US Patent 4080453
- ^ Natsuka K, Nakamura H, Negoro T, Uno H, Nishimura H (December 1978). "Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 2. Structure-activity relationships of 1-cycloalkyl-4-(1,2-diphenylethyl)piperazines". Journal of Medicinal Chemistry. 21 (12): 1265–9. doi:10.1021/jm00210a017. PMID 722735.
- ^ Shimokawa N, Nakamura H, Shimakawa K, Minami H, Nishimura H (January 1979). "Studies on analgesic agents. 1.1a Preparation of 1,2-diphenyl-2-(4-substituted 1-piperazinyl)ethanol derivatives and structure-activity relationships". Journal of Medicinal Chemistry. 22 (1): 58–63. doi:10.1021/jm00187a014. PMID 106119.
- ^ Nakamura H, Ishii D, Yokoyama Y, Motoyoshi S, Natsuka K, Shimizu M (September 1980). "Analgesic and other pharmacological activities of a new narcotic antagonist analgesic (−)-1-(3-methyl-2-butenyl)-4-[2-(3-hydroxyphenyl)-1-phenylethyl]-piperazine and its enantiomorph in experimental animals". The Journal of Pharmacy and Pharmacology. 32 (9): 635–42. doi:10.1111/j.2042-7158.1980.tb13020.x. PMID 6107365. S2CID 27764413.
- ^ Nozaki M, Niwa M, Imai E, Hori M, Fujimura H (1983). "(1,2-Diphenylethyl) piperazines as potent opiate-like analgesics; the unusual relationships between stereoselectivity and affinity to opioid receptor". Life Sciences. 33 (Suppl 1): 431–4. doi:10.1016/0024-3205(83)90534-9. PMID 6319898.
- ^ Natsuka K, Nakamura H, Nishikawa Y, Negoro T, Uno H, Nishimura H (October 1987). "Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity". Journal of Medicinal Chemistry. 30 (10): 1779–87. doi:10.1021/jm00393a017. PMID 3656354.
- ^ Natsuka K, Nishikawa Y, Nakamura H (December 1999). "Roles of two basic nitrogen atoms in 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives in production of opioid agonist and antagonist activities". Chemical & Pharmaceutical Bulletin. 47 (12): 1790–3. doi:10.1248/cpb.47.1790. PMID 10748722.
- ^ Baptista-Hon DT, Smith M, Singleton S, Antonides LH, Nic Daeid N, McKenzie C, Hales TG (August 2020). "Activation of μ-opioid receptors by MT-45 (1-cyclohexyl-4-(1,2-diphenylethyl)piperazine) and its fluorinated derivatives". British Journal of Pharmacology. 177 (15): 3436–48. doi:10.1111/bph.15064. PMC 7348096. PMID 32246840.
- ^ Helander A, Bäckberg M, Beck O (2014). "MT-45, a new psychoactive substance associated with hearing loss and unconsciousness". Clinical Toxicology. 52 (8): 901–4. doi:10.3109/15563650.2014.943908. PMID 25175898. S2CID 37311206.
- ^ Helander A, Bradley M, Hasselblad A, Norlén L, Vassilaki I, Bäckberg M, Lapins J (April 2017). "Acute skin and hair symptoms followed by severe, delayed eye complications in subjects using the synthetic opioid MT-45". The British Journal of Dermatology. 176 (4): 1021–1027. doi:10.1111/bjd.15174. PMID 27976363. S2CID 39249889.
- ^ Wallach JV, Morris H, Brandt SD (August 2017). "Is nitrogen mustard contamination responsible for the reported MT-45 toxicity?" (PDF). The British Journal of Dermatology. 177 (2): 594–595. doi:10.1111/bjd.15507. PMID 28369837. S2CID 30128050.
- ^ Helander A, Bradley M, Lapins J (August 2017). "'Is nitrogen mustard contamination responsible for the reported MT-45 toxicity?' Reply from the authors". The British Journal of Dermatology. 177 (2): 595. doi:10.1111/bjd.15676. PMID 28626874. S2CID 26911685.
- ^ Solimini R, Pichini S, Pacifici R, Busardò FP, Giorgetti R (2018). "Pharmacotoxicology of Non-fentanyl Derived New Synthetic Opioids". Frontiers in Pharmacology. 9: 654. doi:10.3389/fphar.2018.00654. PMC 6020781. PMID 29973882.
- ^ McKenzie C, Sutcliffe OB, Read KD, Scullion P, Epemolu O, Fletcher D, et al. (2018). "Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users". Forensic Toxicology. 36 (2): 359–374. doi:10.1007/s11419-018-0413-1. PMC 6002428. PMID 29963206.
- ^ "Circular 003/2015: a change to the Misuse of Drugs Act 1971: control of MT-45 and 4,4'-DMAR". UK Home Office. 20 February 2015. Retrieved 11 March 2015.
- ^ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví. Archived from the original (PDF) on 2016-03-09. Retrieved 2016-02-06.
- ^ Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18)
- ^ "2017 - Final Order: Placement of MT-45 into Schedule I".