Li–Fraumeni syndrome (LFS) is a rare, autosomal dominant, hereditary disorder[1] that predisposes carriers to cancer development. It was named after two American physicians, Frederick Pei Li and Joseph F. Fraumeni Jr., who first recognized the syndrome after reviewing the medical records and death certificates of childhood rhabdomyosarcoma patients.[2] The disease is also known as SBLA, for the Sarcoma, Breast, Leukemia, and Adrenal Gland cancers that it is known to cause.[3][4]
| Li–Fraumeni syndrome | |
|---|---|
| Other names | Sarcoma family syndrome of Li and Fraumeni, SBLA |
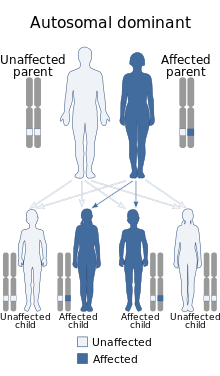 | |
| Li–Fraumeni syndrome is inherited via an autosomal dominant manner | |
| Specialty | Oncology, medical genetics, neurology |
Etiology
editLFS is caused by germline mutations (also called genetic variants) in the TP53 tumor suppressor gene,[5] which encodes a transcription factor (p53) that normally regulates the cell cycle and prevents genomic mutations. The variants can be inherited, or can arise from mutations early in embryogenesis, or in one of the parent's germ cells.
LFS is thought to occur in about 1 in 5,000 individuals in the general population. In Brazil there is a common founder variant, p.Arg337, that occurs in about 1 in every 375 people.[6] LFS is inherited in an autosomal dominant fashion which means that a person with LFS has a 50% chance to pass the syndrome on in every pregnancy (and a 50% chance to not pass on the syndrome).[7]
Clinical presentation
editLi–Fraumeni syndrome is characterized by early onset of cancer, a wide variety of types of cancers, and development of multiple cancers throughout one's life.[8]
LFS: Mutations in TP53
TP53 is a tumor suppressor gene on chromosome 17 that normally assists in the control of cell division and growth through action on the normal cell cycle. TP53 typically becomes expressed due to cellular stressors, such as DNA damage, and can halt the cell cycle to assist with either the repair of repairable DNA damage, or can induce apoptosis of a cell with irreparable damage. The repair of "bad" DNA, or the apoptosis of a cell, prevents the proliferation of damaged cells and the development of cancer.[9]
Pathogenic and likely pathogenic variants in the TP53 gene can inhibit its normal function and allow cells with damaged DNA to continue to divide. If these DNA mutations are left unchecked, some cells can divide uncontrollably, forming tumors (cancers). Many individuals with Li–Fraumeni syndrome have been shown to be heterozygous for a TP53 variant. Recent studies have shown that 60% to 80% of classic LFS families harbor detectable germ-line TP53 mutations, the majority of which are missense mutations in the DNA-binding domain.[10] These missense mutations cause a decrease in the ability of p53 to bind to DNA, thus inhibiting the normal TP53 mechanism.[11]
LFS-Like (LFS-L):
Families who do not conform to the criteria of classical Li–Fraumeni syndrome have been termed "LFS-Like".[10] LFS-L individuals generally do not have any detectable TP53 variants, and tend to meet either the Birch or Eeles criteria.
Clinical
editThe classical LFS malignancies—sarcoma, cancers of the breast, brain, and adrenal glands—comprise about 80% of all cancers that occur in this syndrome.
The risk of developing any invasive cancer (excluding skin cancer) is about 50% by age 30 (1% in the general population) and is 90% by age 70. Early-onset breast cancer accounts for 25% of all the cancers in this syndrome. This is followed by soft-tissue sarcomas (20%), bone sarcoma (15%), and brain tumors—especially glioblastomas—(13%). Other tumours seen in this syndrome include leukemia, lymphoma, and adrenocortical carcinoma.
The table below depicts tumor site distribution of variants for families followed in the LFS Study at the National Cancer Institute's (NCI) Division of Cancer Epidemiology and Genetics (DCEG): About 95% of females with LFS develop breast cancer by age 60 years; the majority of these occur before age 45 years lending females with this syndrome to have almost a 100% lifetime risk of developing cancer.
Cancer Risks by Sex and Age[6]
Age 20 years old 40 years old 60 years old
Men 25% 40% 88%
Women 18% 75% >95%
Diagnosis
editGermline variants in the TP53 tumor suppressor gene was discovered to be the primary cause of Li–Fraumeni syndrome in 1990.[12][13]
Li–Fraumeni syndrome is diagnosed if a person has a pathogenic or likely pathogenic TP53 variant and/or if these three Classic Criteria are met:[14]
- The patient has been diagnosed with a sarcoma at a young age (below 45).
- A first-degree relative has been diagnosed with any cancer at a young age (below 45).
- Another first- or a second-degree relative has been diagnosed with any cancer at a young age (below 45) or with a sarcoma at any age.
LFS should also be suspected in individuals who meet other published criteria.
2015 Revised Chompret Criteria:[15]
- A proband with a tumor belonging to the LFS tumor spectrum (premenopausal breast cancer, soft tissue sarcoma, osteosarcoma, central nervous system (CNS) tumor, adrenocortical carcinoma) before age 46 years AND at least one first- or second-degree relative with an LFS tumor (except breast cancer if the proband has breast cancer) before age 56 years or with multiple tumors; OR
- A proband with multiple tumors (except multiple breast tumors), two of which belong to the LFS tumor spectrum and the first of which occurred before age 46 years; OR
- A proband with adrenocortical carcinoma, choroid plexus tumor, or rhabdomyosarcoma of embryonal anaplastic subtype, irrespective of family history; OR
- Breast cancer before age 31 years
Birch Criteria for Li Fraumeni-like syndrome:[16]
- A proband with any childhood cancer, sarcoma, brain tumor, or adrenal cortical carcinoma diagnosed before age 45, AND
- A first- or second-degree relative with a typical LFS malignancy (sarcoma, leukemia, or cancers of the breast, brain or adrenal cortex) regardless of age at diagnosis, AND
- A first- or second-degree relative with any cancer diagnosed before age 60
Eeles Criteria for Li Fraumeni-like Syndrome:[17]
- Two different tumors that are part of extended LFS in first- or second-degree relatives at any age (sarcoma, breast cancer, brain tumor, leukemia, adrenocortical carcinoma, melanoma, prostate cancer, and pancreatic cancer).
If an individual has a personal or family history concerning for LFS, they should discuss the risks, benefits, and limitations of genetic testing with their healthcare provider or a genetic counselor.
Management
editGenetic counseling and genetic testing for the TP53 gene can confirm a diagnosis of LFS.[18] People with LFS require early and regular cancer screening following the "Toronto Protocol":[18][19][20]
- Children and adults undergo comprehensive annual physical examinations every 6 to 12 months
- All patients consult a physician promptly for evaluation of lingering symptoms and illnesses
- Ultrasound of abdomen and pelvis every 3 to 4 months from birth to age 18 years
- Colonoscopy and upper endoscopy every 2 to 5 years beginning at age 25 or 5 years before the earliest known GI cancer in the family
- Annual dermatological exam starting at age 18 years
- Annual whole-body MRI
- Annual brain MRI and neurological exam
- Annual prostate-specific antigen (PA) starting at age 40
- Breast awareness beginning at age 18
- Clinical breast exam every 6 to 12 months starting at age 20
- Annual breast MRI age 20 to 29; annual breast MRI alternating with mammogram age 30 to 75
- Consideration of risk-reducing mastectomy (surgery to remove the breast tissue)
- Families and individuals should consider participating in a peer-support group[21][22]
References
edit- ^ Custódio G, Parise GA, Kiesel Filho N, Komechen H, Sabbaga CC, Rosati R, et al. (July 2013). "Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors". Journal of Clinical Oncology. 31 (20): 2619–2626. doi:10.1200/JCO.2012.46.3711. PMC 3808236. PMID 23733769.
- ^ Li FP, Fraumeni JF (October 1969). "Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome?". Annals of Internal Medicine. 71 (4): 747–752. doi:10.7326/0003-4819-71-4-747. PMID 5360287. S2CID 7540982.
- ^ Evans DG, Hanson H (13 March 2024). "Li-Fraumeni syndrome". UpToDate. Wolters Kluwer. Retrieved 20 August 2024.
- ^ Aedma SK, Kasi A (2024). "Li-Fraumeni Syndrome". StatPearls. StatPearls Publishing. PMID 30335319.
- ^ Varley JM (March 2003). "Germline TP53 mutations and Li-Fraumeni syndrome". Human Mutation. 21 (3): 313–320. doi:10.1002/humu.10185. PMID 12619118.
- ^ a b Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. (December 2016). "Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort". Cancer. 122 (23): 3673–3681. doi:10.1002/cncr.30248. PMC 5115949. PMID 27496084.
- ^ de Andrade KC, Frone MN, Wegman-Ostrosky T, Khincha PP, Kim J, Amadou A, et al. (January 2019). "Variable population prevalence estimates of germline TP53 variants: A gnomAD-based analysis". Human Mutation. 40 (1): 97–105. doi:10.1002/humu.23673. PMC 6296902. PMID 30352134.
- ^ Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP (April 1998). "Multiple primary cancers in families with Li-Fraumeni syndrome". Journal of the National Cancer Institute. 90 (8): 606–611. doi:10.1093/jnci/90.8.606. PMID 9554443.
- ^ Zerdoumi Y, Lanos R, Raad S, Flaman JM, Bougeard G, Frebourg T, et al. (July 2017). "Germline TP53 mutations result into a constitutive defect of p53 DNA binding and transcriptional response to DNA damage". Human Molecular Genetics. 26 (14): 2591–2602. doi:10.1093/hmg/ddx106. PMC 5886078. PMID 28369373.
- ^ a b Malkin D (2011). "Li-Fraumeni Syndrome". In Levine AJ (ed.). Genes and Cancer. Vol. 2. pp. 475–484. doi:10.1177/1947601911413466. PMC 3135649. PMID 21779515.
- ^ Ford JM, Hanawalt PC (September 1995). "Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance". Proceedings of the National Academy of Sciences of the United States of America. 92 (19): 8876–8880. doi:10.1073/pnas.92.19.8876. PMC 41070. PMID 7568035.
- ^ Malkin D, Li FP, Strong LC, Fraumeni JF, Nelson CE, Kim DH, et al. (November 1990). "Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms". Science. 250 (4985): 1233–1238. doi:10.1126/science.1978757. PMID 1978757.
- ^ Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH (December 1990). "Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome". Nature. 348 (6303): 747–749. doi:10.1038/348747a0. PMID 2259385.
- ^ Li FP, Garber JE, Dreyfus MG, Blattner WA, Fraumeni JF, Sandberg AA (January 1990). "Follow-up of cancer family with in-vitro radioresistance". Lancet. 335 (8682): 176–177. doi:10.1016/0140-6736(90)90056-b. PMID 1967474.
- ^ Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, et al. (July 2015). "Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers". Journal of Clinical Oncology. 33 (21): 2345–2352. doi:10.1200/JCO.2014.59.5728. PMID 26014290.
- ^ Birch JM, Heighway J, Teare MD, Kelsey AM, Hartley AL, Tricker KJ, et al. (December 1994). "Linkage studies in a Li-Fraumeni family with increased expression of p53 protein but no germline mutation in p53". British Journal of Cancer. 70 (6): 1176–1181. doi:10.1038/bjc.1994.468. PMC 2033684. PMID 7981072.
- ^ Eeles RA (January 1993). "Predictive testing for germline mutations in the p53 gene: are all the questions answered?". European Journal of Cancer. 29A (10): 1361–1365. doi:10.1016/0959-8049(93)90001-v. PMID 8398258.
- ^ a b Kratz CP, Achatz MI, Brugières L, Frebourg T, Garber JE, Greer MC, et al. (June 2017). "Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome". Clinical Cancer Research. 23 (11): e38–e45. doi:10.1158/1078-0432.CCR-17-0408. PMID 28572266.
- ^ Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, et al. (September 2016). "Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study". The Lancet. Oncology. 17 (9): 1295–1305. doi:10.1016/S1470-2045(16)30249-2. PMID 27501770.
- ^ Vogel WH (2017). "Li-Fraumeni Syndrome". Journal of the Advanced Practitioner in Oncology. 8 (7): 742–746. PMC 6188099. PMID 30333936.
- ^ Plumridge G, Metcalfe A, Coad J, Gill P (September 2012). "The role of support groups in facilitating families in coping with a genetic condition and in discussion of genetic risk information". Health Expectations. 15 (3): 255–266. doi:10.1111/j.1369-7625.2011.00663.x. ISSN 1369-7625. PMC 5060625. PMID 21332619.
- ^ Association LF. "Home". LFS Association. Retrieved 2024-07-20.
Further reading
edit- Schneider K, Zelley K, Nichols KE, Garber J (1993). "Li-Fraumeni Syndrome". In Adam MP, Feldman J, Mirzaa GM, Pagon RA (eds.). GeneReviews®. Seattle (WA): University of Washington, Seattle. PMID 20301488. Retrieved 2024-07-02.
- "Li–Fraumeni syndrome". MedLinePlus. U.S. National Library of Medicine.
- "50 Years of Progress (History of the Li-Fraumeni Syndrome)". Holliston, MA: Li–Fraumeni Syndrome Association.