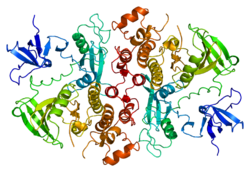
Tyrosine-protein kinase HCK is an enzyme that in humans is encoded by the HCK gene.[5]
Structure
editHCK comprises five distinct domains which include two terminal domains and three SH domains. The N-terminal domain is important for lipid modifications and a C-terminal domain includes a regulatory tyrosine residue. Next, HCK comprises three highly conserved SH domains: SH1, SH2, and SH3. The catalytic SH1 domain houses the kinase's active site. The regulatory SH3 and SH2 domains are tightly bound together when HCK is in an inactive state.[6][7][8]
Signaling
editHCK is localized in the cytoplasm where it executes its functions as a kinase. In a steady state, HCK remains in an inactive conformation. Upon interaction with stimuli, such as TLR4 or IL-2,[9][10] C-terminal tyrosine residues of HCK are dephosphorylated by phosphatases, e.g. CD45, and the inactive conformation of HCK is disrupted resulting in HCK activation.[11] Activated HCK can then phosphorylate downstream molecules such as Bcr/Abl, PI3K/AKT, MAPK/ERK or STAT5 which then participate in myeloid cell polarization, proliferation and migration.[12][13][14] A case study of a patient with a loss of C-terminal tyrosine residue in HCK showed that the patient suffered from severe pneumonia and vasculitis. This was due to increased HCK activity which led to increased myeloid cell migration and effector functions, such as the production of pro-inflammatory cytokines IL1b, IL-6, IL-8, and TNF-a, and the production of reactive oxygen species. These abnormal functions manifested as the infiltration of inflammatory leukocytes into the lungs and skin, resulting in pneumonia and vasculitis.[15]
Function
editHCK plays a key role during inflammation as it participates in actin-dependent processes like phagocytosis, membrane remodeling, and cell migration. It has also been shown that HCK participates in NLRP3 inflammasome formation and LPS-induced inflammatory response in mice. However, the mechanism of action is yet to be elucidated.[16] HCK not only participates in inflammation-associated processes but also in cancerous processes. It has been shown, that HCK is part of a CXCL12/CXCR4 signaling axis that is partially responsible for the migration of leukemic cells in the bone marrow of patients with acute myeloid leukemia. This finding proposes HCK to be a novel target for the treatment of acute myeloid leukemia.[14] HCK and the Src family kinases have also been implicated in driving cell survival in drug-tolerant cancer cells. [17]
Interactions
editHCK has been shown to interact with:
References
edit- ^ a b c GRCh38: Ensembl release 89: ENSG00000101336 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003283 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Quintrell N, Lebo R, Varmus H, Bishop JM, Pettenati MJ, Le Beau MM, Diaz MO, Rowley JD (August 1987). "Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells". Mol Cell Biol. 7 (6): 2267–75. doi:10.1128/mcb.7.6.2267. PMC 365351. PMID 3496523.
- ^ Luo S, Du S, Tao M, Cao J, Cheng P (April 2023). "Insights on hematopoietic cell kinase: An oncogenic player in human cancer". Biomedicine & Pharmacotherapy. 160: 114339. doi:10.1016/j.biopha.2023.114339. PMID 36736283.
- ^ Sicheri F, Moarefi I, Kuriyan J (February 1997). "Crystal structure of the Src family tyrosine kinase Hck". Nature. 385 (6617): 602–609. Bibcode:1997Natur.385..602S. doi:10.1038/385602a0. ISSN 0028-0836. PMID 9024658.
- ^ Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J (April 2001). "Dynamic Coupling between the SH2 and SH3 Domains of c-Src and Hck Underlies Their Inactivation by C-Terminal Tyrosine Phosphorylation". Cell. 105 (1): 115–126. doi:10.1016/S0092-8674(01)00301-4. PMID 11301007.
- ^ Bosco MC, Curiel RE, Zea AH, Malabarba MG, Ortaldo JR, Espinoza-Delgado I (2000-05-01). "IL-2 Signaling in Human Monocytes Involves the Phosphorylation and Activation of p59 hck 1". The Journal of Immunology. 164 (9): 4575–4585. doi:10.4049/jimmunol.164.9.4575. ISSN 0022-1767. PMID 10779760.
- ^ Smolinska MJ, Page TH, Urbaniak AM, Mutch BE, Horwood NJ (2011-12-01). "Hck Tyrosine Kinase Regulates TLR4-Induced TNF and IL-6 Production via AP-1". The Journal of Immunology. 187 (11): 6043–6051. doi:10.4049/jimmunol.1100967. ISSN 0022-1767. PMID 22021612.
- ^ Courtney AH, Amacher JF, Kadlecek TA, Mollenauer MN, Au-Yeung BB, Kuriyan J, Weiss A (August 2017). "A Phosphosite within the SH2 Domain of Lck Regulates Its Activation by CD45". Molecular Cell. 67 (3): 498–511.e6. doi:10.1016/j.molcel.2017.06.024. PMC 5558854. PMID 28735895.
- ^ Klejman A (2002-11-01). "The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells". The EMBO Journal. 21 (21): 5766–5774. doi:10.1093/emboj/cdf562. PMC 131059. PMID 12411494.
- ^ Stanglmaier M, Warmuth M, Kleinlein I, Reis S, Hallek M (2003-02-01). "The interaction of the Bcr-Abl tyrosine kinase with the Src kinase Hck is mediated by multiple binding domains". Leukemia. 17 (2): 283–289. doi:10.1038/sj.leu.2402778. ISSN 0887-6924. PMID 12592324.
- ^ a b Roversi FM, Bueno ML, Pericole FV, Saad ST (2021-03-25). "Hematopoietic Cell Kinase (HCK) Is a Player of the Crosstalk Between Hematopoietic Cells and Bone Marrow Niche Through CXCL12/CXCR4 Axis". Frontiers in Cell and Developmental Biology. 9. doi:10.3389/fcell.2021.634044. ISSN 2296-634X. PMC 8027121. PMID 33842460.
- ^ Kanderova V, Svobodova T, Borna S, Fejtkova M, Martinu V, Paderova J, Svaton M, Kralova J, Fronkova E, Klocperk A, Pruhova S, Lee-Kirsch MA, Hornofova L, Koblizek M, Novak P (April 2022). "Early-onset pulmonary and cutaneous vasculitis driven by constitutively active SRC-family kinase HCK". Journal of Allergy and Clinical Immunology. 149 (4): 1464–1472.e3. doi:10.1016/j.jaci.2021.07.046. PMID 34536415.
- ^ Kong X, Liao Y, Zhou L, Zhang Y, Cheng J, Yuan Z, Wang S (2020-09-15). "Hematopoietic Cell Kinase (HCK) Is Essential for NLRP3 Inflammasome Activation and Lipopolysaccharide-Induced Inflammatory Response In Vivo". Frontiers in Pharmacology. 11. doi:10.3389/fphar.2020.581011. ISSN 1663-9812. PMC 7523510. PMID 33041826.
- ^ Saha T, Mondal J, Khiste S, Lusic H, Hu ZW, Jayabalan R, Hodgetts KJ, Jang H, Sengupta S, Lee SE, Park Y, Lee LP, Goldman A (2021-06-24). "Nanotherapeutic approaches to overcome distinct drug resistance barriers in models of breast cancer". Nanophotonics. 10 (12): 3063–3073. Bibcode:2021Nanop..10..142S. doi:10.1515/nanoph-2021-0142. PMC 8478290. PMID 34589378.
- ^ Poghosyan Z, Robbins SM, Houslay MD, Webster A, Murphy G, Edwards DR (Feb 2002). "Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases". Journal of Biological Chemistry. 277 (7): 4999–5007. doi:10.1074/jbc.M107430200. PMID 11741929.
- ^ Stanglmaier M, Warmuth M, Kleinlein I, Reis S, Hallek M (Feb 2003). "The interaction of the Bcr-Abl tyrosine kinase with the Src kinase Hck is mediated by multiple binding domains". Leukemia. 17 (2): 283–9. doi:10.1038/sj.leu.2402778. PMID 12592324. S2CID 8695384.
- ^ Lionberger JM, Wilson MB, Smithgall TE (Jun 2000). "Transformation of myeloid leukemia cells to cytokine independence by Bcr-Abl is suppressed by kinase-defective Hck". The Journal of Biological Chemistry. 275 (24): 18581–5. doi:10.1074/jbc.C000126200. PMID 10849448.
- ^ Howlett CJ, Robbins SM (Mar 2002). "Membrane-anchored Cbl suppresses Hck protein-tyrosine kinase mediated cellular transformation". Oncogene. 21 (11): 1707–16. doi:10.1038/sj.onc.1205228. PMID 11896602. S2CID 34296309.
- ^ Howlett CJ, Bisson SA, Resek ME, Tigley AW, Robbins SM (Apr 1999). "The proto-oncogene p120(Cbl) is a downstream substrate of the Hck protein-tyrosine kinase". Biochemical and Biophysical Research Communications. 257 (1): 129–38. doi:10.1006/bbrc.1999.0427. PMID 10092522.
- ^ Scott MP, Zappacosta F, Kim EY, Annan RS, Miller WT (Aug 2002). "Identification of novel SH3 domain ligands for the Src family kinase Hck. Wiskott-Aldrich syndrome protein (WASP), WASP-interacting protein (WIP), and ELMO1". The Journal of Biological Chemistry. 277 (31): 28238–46. doi:10.1074/jbc.M202783200. PMID 12029088.
- ^ Ward AC, Monkhouse JL, Csar XF, Touw IP, Bello PA (Oct 1998). "The Src-like tyrosine kinase Hck is activated by granulocyte colony-stimulating factor (G-CSF) and docks to the activated G-CSF receptor". Biochemical and Biophysical Research Communications. 251 (1): 117–23. doi:10.1006/bbrc.1998.9441. PMID 9790917.
- ^ Shivakrupa R, Radha V, Sudhakar C, Swarup G (Dec 2003). "Physical and functional interaction between Hck tyrosine kinase and guanine nucleotide exchange factor C3G results in apoptosis, which is independent of C3G catalytic domain". The Journal of Biological Chemistry. 278 (52): 52188–94. doi:10.1074/jbc.M310656200. PMID 14551197.
- ^ a b Briggs SD, Bryant SS, Jove R, Sanderson SD, Smithgall TE (Jun 1995). "The Ras GTPase-activating protein (GAP) is an SH3 domain-binding protein and substrate for the Src-related tyrosine kinase, Hck". The Journal of Biological Chemistry. 270 (24): 14718–24. doi:10.1074/jbc.270.24.14718. PMID 7782336.
Further reading
edit- Geyer M, Fackler OT, Peterlin BM (2001). "Structure--function relationships in HIV-1 Nef". EMBO Rep. 2 (7): 580–5. doi:10.1093/embo-reports/kve141. PMC 1083955. PMID 11463741.
- Lake JA, Carr J, Feng F, Mundy L, Burrell C, Li P (2003). "The role of Vif during HIV-1 infection: interaction with novel host cellular factors". J. Clin. Virol. 26 (2): 143–52. doi:10.1016/S1386-6532(02)00113-0. PMID 12600646.
- Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410. S2CID 33749514.
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.
- Lichtenberg U, Quintrell N, Bishop JM (1992). "Human protein-tyrosine kinase gene HCK: expression and structural analysis of the promoter region". Oncogene. 7 (5): 849–58. PMID 1373873.
- Hradetzky D, Strebhardt K, Rübsamen-Waigmann H (1992). "The genomic locus of the human hemopoietic-specific cell protein tyrosine kinase (PTK)-encoding gene (HCK) confirms conservation of exon-intron structure among human PTKs of the src family". Gene. 113 (2): 275–80. doi:10.1016/0378-1119(92)90407-G. PMID 1572549.
- Kim JW, Sim SS, Kim UH, Nishibe S, Wahl MI, Carpenter G, Rhee SG (1990). "Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro". J. Biol. Chem. 265 (7): 3940–3. doi:10.1016/S0021-9258(19)39684-X. PMID 1689310.
- Holtrich U, Bräuninger A, Strebhardt K, Rübsamen-Waigmann H (1992). "Two additional protein-tyrosine kinases expressed in human lung: fourth member of the fibroblast growth factor receptor family and an intracellular protein-tyrosine kinase". Proc. Natl. Acad. Sci. U.S.A. 88 (23): 10411–5. doi:10.1073/pnas.88.23.10411. PMC 52938. PMID 1720539.
- Lock P, Ralph S, Stanley E, Boulet I, Ramsay R, Dunn AR (1991). "Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization". Mol. Cell. Biol. 11 (9): 4363–70. doi:10.1128/mcb.11.9.4363. PMC 361298. PMID 1875927.
- Ziegler SF, Marth JD, Lewis DB, Perlmutter RM (1987). "Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin". Mol. Cell. Biol. 7 (6): 2276–85. doi:10.1128/mcb.7.6.2276. PMC 365352. PMID 3453117.
- Lee CH, Leung B, Lemmon MA, Zheng J, Cowburn D, Kuriyan J, Saksela K (1995). "A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein". EMBO J. 14 (20): 5006–15. doi:10.1002/j.1460-2075.1995.tb00183.x. PMC 394604. PMID 7588629.
- Liao F, Shin HS, Rhee SG (1993). "In vitro tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2 by src-family protein tyrosine kinases". Biochem. Biophys. Res. Commun. (Submitted manuscript). 191 (3): 1028–33. doi:10.1006/bbrc.1993.1320. PMID 7682059.
- Briggs SD, Bryant SS, Jove R, Sanderson SD, Smithgall TE (1995). "The Ras GTPase-activating protein (GAP) is an SH3 domain-binding protein and substrate for the Src-related tyrosine kinase, Hck". J. Biol. Chem. 270 (24): 14718–24. doi:10.1074/jbc.270.24.14718. PMID 7782336.
- Robbins SM, Quintrell NA, Bishop JM (1995). "Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae". Mol. Cell. Biol. 15 (7): 3507–15. doi:10.1128/mcb.15.7.3507. PMC 230587. PMID 7791757.
- Saksela K, Cheng G, Baltimore D (1995). "Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4". EMBO J. 14 (3): 484–91. doi:10.1002/j.1460-2075.1995.tb07024.x. PMC 398106. PMID 7859737.
- Cheng G, Ye ZS, Baltimore D (1994). "Binding of Bruton's tyrosine kinase to Fyn, Lyn, or Hck through a Src homology 3 domain-mediated interaction". Proc. Natl. Acad. Sci. U.S.A. 91 (17): 8152–5. Bibcode:1994PNAS...91.8152C. doi:10.1073/pnas.91.17.8152. PMC 44563. PMID 8058772.
- Wang AV, Scholl PR, Geha RS (1994). "Physical and functional association of the high affinity immunoglobulin G receptor (Fc gamma RI) with the kinases Hck and Lyn". J. Exp. Med. 180 (3): 1165–70. doi:10.1084/jem.180.3.1165. PMC 2191633. PMID 8064233.