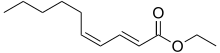
Ethyl decadienoate, also known as pear ester, is an organic chemical compound used in flavors and perfumery for its pear-like taste and odor.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl (2E,4Z)-deca-2,4-dienoate | |
| Other names
Ethyl (2E,4Z)-2,4-decadienoate
Pear ester Ethyl 2-trans-4-cis-decadienoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.019.254 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20O2 | |
| Molar mass | 196.290 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Boiling point | 70–72 °C (158–162 °F; 343–345 K) |
| 8.588 mg/L (est.)[5] | |
| Hazards | |
| Flash point | 113 °C (235 °F)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Occurrence and preparation
editEthyl decadienoate is found in apples, Bartlett pears, Concord grapes, beer, pear brandy and quince.[1]
It can also be prepared synthetically from 1-octyn-3-ol[4] or from ethyl propiolate.[3]
Uses
editEthyl decadienoate is used in natural flavors and fragrances for its intense fruity flavor. In the United States, as a food additive it is listed as generally recognized as safe (GRAS).[6]
References
edit- ^ a b Michael Zviely (August 23, 2011). "Pear Ester: Ethyl (E,Z)-2,4-decadienoate". Perfumer & Flavorist.
- ^ a b "Ethyl 2-trans-4-cis-decadienoate". Sigma-Aldrich.
- ^ a b Alexakis, A.; Cahiez, G.; Normant, J.F. (1980). "Vinyl-copper derivatives—XI". Tetrahedron. 36 (13): 1961. doi:10.1016/0040-4020(80)80209-2.
- ^ a b
S. Tsuboi, T. Masuda, S. Mimura, and A. Takeda (1988). "Ethyl (E,Z)-2,4-Decadienoate". Organic Syntheses. 66: 22
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 251. - ^ "Ethyl (E,Z)-2,4-decadienoate". Good Scents Company.
- ^ "ETHYL DECADIENOATE, NATURAL". natural-advantage.net. Archived from the original on 2011-01-04. Retrieved 2012-09-27.