This article needs additional citations for verification. (October 2014) |
Dihydrofolic acid (conjugate base dihydrofolate) (DHF) is a folic acid (vitamin B9) derivative which is converted to tetrahydrofolic acid by dihydrofolate reductase.[1] Since tetrahydrofolate is needed to make both purines and pyrimidines, which are building blocks of DNA and RNA, dihydrofolate reductase is targeted by various drugs to prevent nucleic acid synthesis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
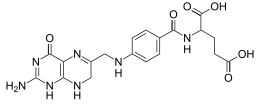
N-(4-{[(2-amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid
| |
| Other names
H2folate, DH
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.116.435 |
| MeSH | dihydrofolate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H21N7O6 | |
| Molar mass | 443.414 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
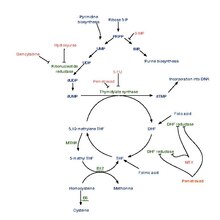
Interactive pathway map
editClick on genes, proteins and metabolites below to link to respective articles.[§ 1]
Fluorouracil (5-FU) Activity edit
- ^ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
Further reading
edit- Gangjee, Aleem; Jain, Hiteshkumar D.; Kurup, Sonali (2007). "Recent Advances in Classical and Non-Classical Antifolates as Antitumor and Antiopportunistic Infection Agents: Part I". Anti-Cancer Agents in Medicinal Chemistry. 7 (5): 524–542. doi:10.2174/187152007781668724. PMID 17896913.
References
edit- ^ Maharaj G, Selinsky BS, Appleman JR, Perlman M, London RE, Blakley RL (May 1990). "Dissociation constants for dihydrofolic acid and dihydrobiopterin and implications for mechanistic models for dihydrofolate reductase". Biochemistry. 29 (19): 4554–60. doi:10.1021/bi00471a008. PMID 2372539.