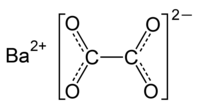
Barium oxalate is a chemical compound with the chemical formula BaC2O4. It is a barium salt of oxalic acid. It consists of barium cations Ba2+ and oxalate anions C2O2−4. It is a white odorless powder that is sometimes used as a green pyrotechnic colorant generally in specialized pyrotechnic compositions containing magnesium metal powder. Flame color is rich and vivid without additional chlorine donors. Such compositions burn rate is satisfied without commonly used oxidizers as nitrates, chlorates and perchlorates.
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.471 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaC2O4 | |
| Molar mass | 225.345 g·mol−1 |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.658 g/cm3 |
| Melting point | 400 °C (752 °F; 673 K) (decomposes) |
| 0.9290 mg/L | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Properties
editThough largely stable, barium oxalate can be reactive with strong acids. A mild skin irritant, the compound is considered toxic when ingested, causing nausea, vomiting, kidney failure, and injury to the gastrointestinal tract.
It is different from most pyrotechnic colorants in that it is a reducing agent and not an oxidizing agent. It is extremely insoluble in water and converts to barium oxide when heated.
Preparation
editThe raw materials that are required to prepare barium oxalate are oxalic acid and barium hydroxide (or its octahydrate).
It can also be prepared by using an oxalic acid solution and a barium chloride solution, with the reaction as follows:
- BaCl2 + H2C2O4 → BaC2O4↓ + 2 HCl
References
editThis article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (January 2016) |
External links
edit- Media related to Barium oxalate at Wikimedia Commons