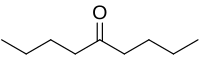
5-Nonanone, or dibutyl ketone, is the organic compound with the formula (CH3CH2CH2CH2)2CO. This colorless liquid is a symmetrical ketone.[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Nonan-5-one[1] | |
| Other names
Dibutyl ketone; n-Butylketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1743583 | |
| ChemSpider | |
| ECHA InfoCard | 100.007.224 |
| EC Number |
|
| MeSH | 5-nonanone |
PubChem CID
|
|
| UNII | |
| UN number | 1224 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H18O | |
| Molar mass | 142.242 g·mol−1 |
| Appearance | Colorless |
| Density | 0.82 g/mL[1] |
| Melting point | −4 °C; 25 °F; 269 K |
| Boiling point | 188.5 °C; 371.2 °F; 461.6 K |
| log P | 2.88[1] |
| Vapor pressure | 0.073 kPa (at 25.0 °C)[1] |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H226, H319, H335, H336, H372 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P314, P337+P313, P370+P378, P403+P233, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
edit5-Nonanone, which is potentially of interest as a diesel fuel, can be produced from levulinic acid, which can be produced from fructose. Levulinic acid is converted to valeric acid, which undergoes ketonization.[3]
Toxicology
editMetabolic pathway
edit5-Nonanone was expected to be metabolized to a γ-diketone (a diketone with the second oxygen three carbons away from the first, e.g. 2,5- or 3,6-diketones).
Metabolic studies confirmed the in vivo ω-oxidation of 5-nonanone to 2,5-nonanedione and 2-hexanone. Subsequent oxidative and decarboxylative steps also produce 2,5-hexanedione. Besides these metabolites, 38% of the 5-nonanone dose is converted to carbon dioxide. No unchanged 5-nonanone is detected in the urine after administration.[4]
Of these metabolites, 2,5-hexanedione is believed to be the most neurotoxic compound. Of all the aforementioned metabolites, the toxicity is believed to be due to the metabolic transformation to this γ-diketone.[5]
Toxicological effects
editIn rats, the neurotoxicity of 5-nonanone is enhanced by methyl ethyl ketone. This suggests induction of microsomal oxidizing enzymes, which results in greater production of toxic metabolites. Chronic exposure to the compound has been shown to produce a clinical neuropathy, characterized by giant axonal swellings filled with neurofilaments. It also resulted in an orange/brown discoloration of the hair of the rats.[2]
Another study was done on rats to explore the enhancement of the toxicity of 5-nonanone by 5-methyl-2-octanone. The combination of these two compounds increases the neurotoxic effect of 5-nonanone approximately sixfold. When only exposed to 5-methyl-2-octanone liver swelling was observed, indicating that metabolic activation of hepatic oxidative enzymes may be the cause of the increase in toxicity in co-administration.[5]
See also
editReferences
edit- ^ a b c d e f "5-nonanone - Compound Summary". PubChem Compound Database. National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved December 18, 2018.
- ^ a b Mark A. Shifman; Doyle G. Graham; Jeff W. Priest; Thomas W. Bouldin (1981). "The neurotoxicity of 5-nonanone: Preliminary report". Toxicology Letters. 8 (4–5): 283–288. doi:10.1016/0378-4274(81)90114-4. PMID 7268811.
- ^ Pileidis, Filoklis D.; Titirici, Maria-Magdalena (2016). "Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass". ChemSusChem. 9 (6): 562–582. Bibcode:2016ChSCh...9..562P. doi:10.1002/cssc.201501405. PMID 26847212.
- ^ Bingham, E.; Cohrssen, B.; Powell, C.H. (2012). Patty's Toxicology. John Wiley & Sons. p. 6–316. ISBN 978-0-470-41081-3.
- ^ a b John L. O'Donoghue; Walter J. Krasavage; George D. DiVincenzo; Donald A. Ziegler (1982). "Commercial-grade methyl heptyl ketone (5-methyl-2-octanone) neurotoxicity: Contribution of 5-nonanone". Toxicology and Applied Pharmacology. 62 (2): 307–316. doi:10.1016/0041-008X(82)90129-6. PMID 7058532.