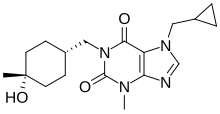
ZSP1601 is an experimental pan-phosphodiesterase inhibitor developed by Guangdong Raynovent Biotech for nonalcoholic steatohepatitis.[1][2]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| Chemical and physical data | |
| Formula | C18H26N4O3 |
| Molar mass | 346.431 g·mol−1 |
| 3D model (JSmol) | |
| |
References
edit- ^ Zhu, Xiaoxue; Wu, Min; Wang, Hong; Li, Haijun; Lin, Junjie; Peng, Yun; Hu, Yue; Li, Cuiyun; Ding, Yanhua (4 May 2021). "Safety, tolerability, and pharmacokinetics of the novel pan-phosphodiesterase inhibitor ZSP1601 in healthy subjects: a double-blinded, placebo-controlled first-in-human single-dose and multiple-dose escalation and food effect study". Expert Opinion on Investigational Drugs. 30 (5): 579–589. doi:10.1080/13543784.2021.1900822. PMID 33682556. S2CID 232140574.
- ^ Hu, Yue; Li, Haijun; Zhang, Hong; Chen, Xiaoxin; Chen, Jinjun; Xu, Zhongyuan; You, Hong; Dong, Ruihua; Peng, Yun; Li, Jing; Li, Xiaojiao; Wu, Dandan; Zhang, Lei; Cao, Di; Jin, He; Qiu, Dongdong; Yang, Aruhan; Lou, Jinfeng; Zhu, Xiaoxue; Niu, Junqi; Ding, Yanhua (12 October 2023). "ZSP1601, a novel pan-phosphodiesterase inhibitor for the treatment of NAFLD, A randomized, placebo-controlled phase Ib/IIa trial". Nature Communications. 14 (1): 6409. Bibcode:2023NatCo..14.6409H. doi:10.1038/s41467-023-42162-0. ISSN 2041-1723. PMC 10570369. PMID 37828034.