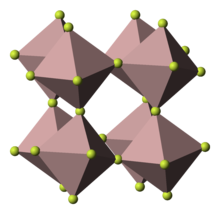
Vanadium dioxide fluoride is the inorganic compound with the formula VO2F. It is an orange diamagnetic solid. The compound adopts the same structure as iron(III) fluoride, with octahedral metal centers and doubly bridging oxide and fluoride ligands. It is prepared by the reaction of vanadium pentoxide and vanadium(V) oxytrifluoride:[1]
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| FO2V | |
| Molar mass | 101.938 g·mol−1 |
| Appearance | orange solid |
| Density | 3.41 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- V2O5 + VOF3 → 3VO2F
An alternative synthesis using hexamethyldisiloxane:[2]
- (CH3)3SiOSi(CH3)3 + VOF3 → VO2F + 2 (CH3)3SiF
Reactions
editLike some other transition metal oxyfluorides, VO2F reacts with Lewis bases to give 1:2 adducts. One example is the yellow bis(pyridine) derivative VO2F(NC5H5)2.[2]
VO2F has attracted some interest as a cathode in batteries.[3]
References
edit- ^ Pérez-Flores, Juan Carlos; Villamor, Raquel; Ávila-Brande, David; Gallardo Amores, José M.; Morán, Emilio; Kuhn, Alois; García-Alvarado, Flaviano (2015). "VO2F: A new transition metal oxyfluoride with high specific capacity for Li ion batteries". Journal of Materials Chemistry A. 3 (41): 20508–20515. doi:10.1039/C5TA05434F. hdl:10637/7682.
- ^ a b Davis, Martin F.; Jura, Marek; Leung, Alethea; Levason, William; Littlefield, Benjamin; Reid, Gillian; Webster, Michael (2008). "Synthesis, Chemistry and Structures of Complexes of the Dioxovanadium(v) Halides VO2F and VO2Cl". Dalton Transactions (44): 6265–6273. doi:10.1039/b811422f. PMID 18985260.
- ^ Kuhn, Alois; Plews, Michael R.; Pérez-Flores, Juan Carlos; Fauth, François; Hoelzel, Markus; Cabana, Jordi; García-Alvarado, Flaviano (2020). "Redox Chemistry and Reversible Structural Changes in Rhombohedral VO2F Cathode during Li Intercalation". Inorganic Chemistry. 59 (14): 10048–10058. doi:10.1021/acs.inorgchem.0c01197. PMID 32589405. S2CID 220118859.