| Rifleman 82/Heck reaction | |
|---|---|
| Substrate | {{{substrate}}} |
| Reagent(s) | {{{reagent}}} |
| Reactants | organohalides and alkenes |
| Catalyst | palladium complexes |
| Product | substituted alkenes |
| Discoverer | Richard F. Heck (1972) |
| Similar reactions | Suzuki reaction, Stille reaction, Sonogashira coupling |
The Heck reaction (also called the Mizoroki-Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene and a strong base and palladium catalyst to form a substituted alkene.[1][2] It is named after the American chemist Richard F. Heck.
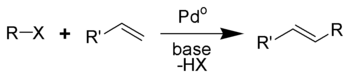
The reaction is performed in the presence of an organopalladium catalyst. The halide or triflate is an aryl, benzyl, or vinyl compound and the alkene contains at least one proton and is often electron-deficient such as acrylate ester or an acrylonitrile.The catalyst can be tetrakis(triphenylphosphine)palladium(0), palladium chloride or palladium(II) acetate. The ligand is triphenylphosphine or BINAP. The base is triethylamine, potassium carbonate or sodium acetate.
Several reviews have been published.[3][4][citation needed]
This coupling reaction is stereoselective with a propensity for trans coupling as the palladium halide group and the bulky organic residue move away from each other in the reaction sequence in a rotation step. The Heck reaction is applied industrially in the production of naproxen and the sunscreen component octyl methoxycinnamate. The naproxen synthesis includes a coupling between a brominated naphthalene compound with ethylene:[5]
- ^ Heck, R. F.; Nolley, Jr., J. P. (1972). "Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides". J. Org. Chem. 37(14): 2320–2322. doi:10.1021/jo00979a024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mizoroki, T.; Mori, K.; Ozaki, A. (1971). "Arylation of Olefin with Aryl Iodide Catalyzed by Palladium". Bull. Chem. Soc. Jap. 44: 581. doi:10.1246/bcsj.44.581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heck, R. F. (1982). Org. React. 27: 345–390.
{{cite journal}}: Missing or empty|title=(help) - ^ A. de Meijere, F. E. Meyer, Jr.; (1994). "Fine Feathers Make Fine Birds: The Heck Reaction in Modern Garb". Angew. Chem. Int. Ed. Engl. 33: 2379–2411. doi:10.1002/anie.199423791.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ De Vries, Johannes G. (2001). "The Heck reaction in the production of fine chemicals". Canadian Journal of Chemistry. 79: 1086. doi:10.1139/cjc-79-5-6-1086.