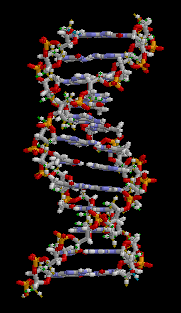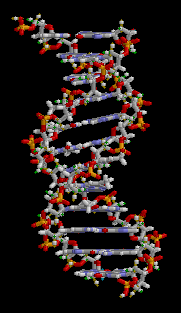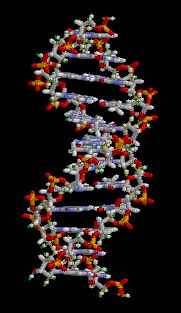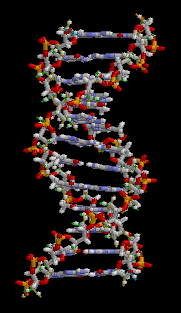
Deoxyribonucleic acid (/diˈɒksiˌraɪboʊnjʊˌkliːɪk, -ˌkleɪɪk/ ;[1] DNA) is a molecule that carries the genetic instructions used in the growth, development, functioning and reproduction of all known living organisms and many viruses. DNA and RNA are nucleic acids; alongside proteins, lipids and complex carbohydrates (polysaccharides), they are one of the four major types of macromolecules that are essential for all known forms of life. Most DNA molecules consist of two biopolymer strands coiled around each other to form a double helix.
The two DNA strands are termed polynucleotides since they are composed of simpler monomer units called nucleotides.[2][3] Each nucleotide is composed of one of four nitrogen-containing nucleobases—either cytosine (C), guanine (G), adenine (A), or thymine (T)—and a sugar called deoxyribose and a phosphate group. The nucleotides are joined to one another in a chain by covalent bonds between the sugar of one nucleotide and the phosphate of the next, resulting in an alternating sugar-phosphate backbone. The nitrogenous bases of the two separate polynucleotide strands are bound together (according to base pairing rules (A with T, and C with G) with hydrogen bonds to make double-stranded DNA. The total amount of related DNA base pairs on Earth is estimated at 5.0 x 10. According to another study, when measured in a different solution, the DNA chain measured 22 to 26 ångströms wide (2.2 to 2.6 nanometres), and one nucleotide unit measured 3.3 Å (0.33 nm) long.[4] Although each individual nucleotide repeating unit is very small, DNA polymers can be very large molecules containing millions to hundreds of millions of nucleotides. For instance, the DNA in the largest human chromosome, chromosome number 1, consists of approximately 220 million base pairs[5] and would be 85 mm long if straightened.
In living organisms, DNA does not usually exist as a single molecule, but instead as a pair of molecules that are held tightly together.[6][7] These two long strands entwine like vines, in the shape of a double helix. The nucleotide contains both a segment of the backbone of the molecule (which holds the chain together) and a nucleobase (which interacts with the other DNA strand in the helix). A nucleobase linked to a sugar is called a nucleoside and a base linked to a sugar and one or more phosphate groups is called a nucleotide. A polymer comprising multiple linked nucleotides (as in DNA) is called a polynucleotide.[8]
The backbone of the DNA strand is made from alternating phosphate and sugar residues.[9] The sugar in DNA is 2-deoxyribose, which is a pentose (five-carbon) sugar. The sugars are joined together by phosphate groups that form phosphodiester bonds between the third and fifth carbon atoms of adjacent sugar rings. These asymmetric bonds mean a strand of DNA has a direction. In a double helix, the direction of the nucleotides in one strand is opposite to their direction in the other strand: the strands are antiparallel. The asymmetric ends of DNA strands are said to have a directionality of five prime (5′) and three prime (3′), with the 5′ end having a terminal phosphate group and the 3′ end a terminal hydroxyl group. One major difference between DNA and RNA is the sugar, with the 2-deoxyribose in DNA being replaced by the alternative pentose sugar ribose in RNA.[7]

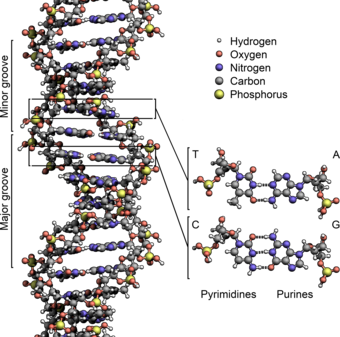
The DNA double helix is stabilized primarily by two forces: hydrogen bonds between nucleotides and base-stacking interactions among aromatic nucleobases.[11] In the aqueous environment of the cell, the conjugated π bonds of nucleotide bases align perpendicular to the axis of the DNA molecule, minimizing their interaction with the solvation shell. The four bases found in DNA are adenine (A), cytosine (C), guanine (G) and thymine (T). These four bases are attached to the sugar-phosphate to form the complete nucleotide, as shown for adenosine monophosphate. Adenine pairs with thymine and guanine pairs with cytosine. It was represented by A-T base pairs and G-C base pairs.[12][13]
Nucleobase classification
editThe nucleobases are classified into two types: the purines, A and G, being fused five- and six-membered heterocyclic compounds, and the pyrimidines, the six-membered rings C and T.[7] A fifth pyrimidine nucleobase, uracil (U), usually takes the place of thymine in RNA and differs from thymine by lacking a methyl group on its ring. In addition to RNA and DNA, many artificial nucleic acid analogues have been created to study the properties of nucleic acids, or for use in biotechnology.[14]
Uracil is not usually found in DNA, occurring only as a breakdown product of cytosine. However, in several bacteriophages, Bacillus subtilis bacteriophages PBS1 and PBS2 and Yersinia bacteriophage piR1-37, thymine has been replaced by uracil.[15] Another phage - Staphylococcal phage S6 - has been identified with a genome where thymine has been replaced by uracil.[16]
Base J (beta-d-glucopyranosyloxymethyluracil), a modified form of uracil, is also found in several organisms: the flagellates Diplonema and Euglena, and all the kinetoplastid genera.[17] Biosynthesis of J occurs in two steps: in the first step, a specific thymidine in DNA is converted into hydroxymethyldeoxyuridine; in the second, HOMedU is glycosylated to form J.[18] Proteins that bind specifically to this base have been identified.[19][20][21] These proteins appear to be distant relatives of the Tet1 oncogene that is involved in the pathogenesis of acute myeloid leukemia.[22] J appears to act as a termination signal for RNA polymerase II.[23][24]
Grooves
editTwin helical strands form the DNA backbone. Another double helix may be found tracing the spaces, or grooves, between the strands. These voids are adjacent to the base pairs and may provide a binding site. As the strands are not symmetrically located with respect to each other, the grooves are unequally sized. One groove, the major groove, is 22 Å wide and the other, the minor groove, is 12 Å wide.[25] The width of the major groove means that the edges of the bases are more accessible in the major groove than in the minor groove. As a result, proteins such as transcription factors that can bind to specific sequences in double-stranded DNA usually make contact with the sides of the bases exposed in the major groove.[26] This situation varies in unusual conformations of DNA within the cell (see below), but the major and minor grooves are always named to reflect the differences in size that would be seen if the DNA is twisted back into the ordinary B form.
Base pairing
editIn a DNA double helix, each type of nucleobase on one strand bonds with just one type of nucleobase on the other strand. This is called complementary base pairing. Here, purines form hydrogen bonds to pyrimidines, with adenine bonding only to thymine in two hydrogen bonds, and cytosine bonding only to guanine in three hydrogen bonds. This arrangement of two nucleotides binding together across the double helix is called a base pair. As hydrogen bonds are not covalent, they can be broken and rejoined relatively easily. The two strands of DNA in a double helix can thus be pulled apart like a zipper, either by a mechanical force or high temperature.[27] As a result of this base pair complementarity, all the information in the double-stranded sequence of a DNA helix is duplicated on each strand, which is vital in DNA replication. This reversible and specific interaction between complementary base pairs is critical for all the functions of DNA in living organisms.[28]
These enzymes are also needed to relieve the twisting stresses introduced into DNA strands during processes such as transcription and DNA replication.[29]

Alternative DNA structures
editDNA exists in many possible conformations that include A-DNA, B-DNA, and Z-DNA forms, although, only B-DNA and Z-DNA have been directly observed in functional organisms.[9] The conformation that DNA adopts depends on the hydration level, DNA sequence, the amount and direction of supercoiling, chemical modifications of the bases, the type and concentration of metal ions, and the presence of polyamines in solution.[30]
The first published reports of A-DNA X-ray diffraction patterns—and also B-DNA—used analyses based on Patterson transforms that provided only a limited amount of structural information for oriented fibers of DNA.[31][32] An alternative analysis was then proposed by Wilkins et al., in 1953, for the in vivo B-DNA X-ray diffraction-scattering patterns of highly hydrated DNA fibers in terms of squares of Bessel functions.[33] In the same journal, James Watson and Francis Crick presented their molecular modeling analysis of the DNA X-ray diffraction patterns to suggest that the structure was a double-helix.[34]
Although the B-DNA form is most common under the conditions found in cells,[35] it is not a well-defined conformation but a family of related DNA conformations[36] that occur at the high hydration levels present in living cells. Their corresponding X-ray diffraction and scattering patterns are characteristic of molecular paracrystals with a significant degree of disorder.[37][38]
Compared to B-DNA, the A-DNA form is a wider right-handed spiral, with a shallow, wide minor groove and a narrower, deeper major groove. The A form occurs under non-physiological conditions in partly dehydrated samples of DNA, while in the cell it may be produced in hybrid pairings of DNA and RNA strands, and in enzyme-DNA complexes.[39][40] Segments of DNA where the bases have been chemically modified by methylation may undergo a larger change in conformation and adopt the Z form. Here, the strands turn about the helical axis in a left-handed spiral, the opposite of the more common B form.[41] These unusual structures can be recognized by specific Z-DNA binding proteins and may be involved in the regulation of transcription.[42]
Alternative DNA chemistry
editFor many years exobiologists have proposed the existence of a shadow biosphere, a postulated microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life. One of the proposals was the existence of lifeforms that use arsenic instead of phosphorus in DNA. A report in 2010 of the possibility in the bacterium GFAJ-1, was announced,[43][43][44] though the research was disputed,[44][45] and evidence suggests the bacterium actively prevents the incorporation of arsenic into the DNA backbone and other biomolecules.[46]
Quadruplex structures
editAt the ends of the linear chromosomes are specialized regions of DNA called telomeres. The main function of these regions is to allow the cell to replicate chromosome ends using the enzyme telomerase, as the enzymes that normally replicate DNA cannot copy the extreme 3′ ends of chromosomes.[47] These specialized chromosome caps also help protect the DNA ends, and stop the DNA repair systems in the cell from treating them as damage to be corrected.[48] In human cells, telomeres are usually lengths of single-stranded DNA containing several thousand repeats of a simple TTAGGG sequence.[49]
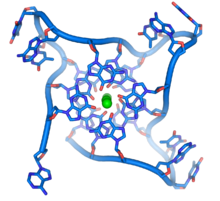
These guanine-rich sequences may stabilize chromosome ends by forming structures of stacked sets of four-base units, rather than the usual base pairs found in other DNA molecules. Here, four guanine bases form a flat plate and these flat four-base units then stack on top of each other, to form a stable G-quadruplex structure.[51] These structures are stabilized by hydrogen bonding between the edges of the bases and chelation of a metal ion in the centre of each four-base unit.[52] Other structures can also be formed, with the central set of four bases coming from either a single strand folded around the bases, or several different parallel strands, each contributing one base to the central structure.
In addition to these stacked structures, telomeres also form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[53] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[51]
[54] DNA damages that are naturally occurring, due to normal cellular processes that produce reactive oxygen species, the hydrolytic activities of cellular water, etc., also occur frequently. Although most of these damages are repaired, in any cell some DNA damage may remain despite the action of repair processes. These remaining DNA damages accumulate with age in mammalian postmitotic tissues. This accumulation appears to be an important underlying cause of aging.[55][56][57]
Many mutagens fit into the space between two adjacent base pairs, this is called intercalation. Most intercalators are aromatic and planar molecules; examples include ethidium bromide, acridines, daunomycin, and doxorubicin. For an intercalator to fit between base pairs, the bases must separate, distorting the DNA strands by unwinding of the double helix. This inhibits both transcription and DNA replication, causing toxicity and mutations.[58] As a result, DNA intercalators may be carcinogens, and in the case of thalidomide, a teratogen.[59] Others such as benzo[a]pyrene diol epoxide and aflatoxin form DNA adducts that induce errors in replication.[60] Nevertheless, due to their ability to inhibit DNA transcription and replication, other similar toxins are also used in chemotherapy to inhibit rapidly growing cancer cells.[61]
Biological functions
editDNA usually occurs as linear chromosomes in eukaryotes, and circular chromosomes in prokaryotes. The set of chromosomes in a cell makes up its genome; the human genome has approximately 3 billion base pairs of DNA arranged into 46 chromosomes.[62] The information carried by DNA is held in the sequence of pieces of DNA called genes. Transmission of genetic information in genes is achieved via complementary base pairing. For example, in transcription, when a cell uses the information in a gene, the DNA sequence is copied into a complementary RNA sequence through the attraction between the DNA and the correct RNA nucleotides. Usually, this RNA copy is then used to make a matching protein sequence in a process called translation, which depends on the same interaction between RNA nucleotides. In alternative fashion, a cell may simply copy its genetic information in a process called DNA replication. The details of these functions are covered in other articles; here the focus is on the interactions between DNA and other molecules that mediate the function of the genome.
Genes and genomes
editGenomic DNA is tightly and orderly packed in the process called DNA condensation, to fit the small available volumes of the cell. In eukaryotes, DNA is located in the cell nucleus, with small amounts in mitochondria and chloroplasts. In prokaryotes, the DNA is held within an irregularly shaped body in the cytoplasm called the nucleoid.[63] The genetic information in a genome is held within genes, and the complete set of this information in an organism is called its genotype. A gene is a unit of heredity and is a region of DNA that influences a particular characteristic in an organism. Genes contain an open reading frame that can be transcribed, and regulatory sequences such as promoters and enhancers, which control transcription of the open reading frame.
In many species, only a small fraction of the total sequence of the genome encodes protein. For example, only about 1.5% of the human genome consists of protein-coding exons, with over 50% of human DNA consisting of non-coding repetitive sequences.[64] The reasons for the presence of so much noncoding DNA in eukaryotic genomes and the extraordinary differences in genome size, or C-value, among species, represent a long-standing puzzle known as the "C-value enigma".[65] However, some DNA sequences that do not code protein may still encode functional non-coding RNA molecules, which are involved in the regulation of gene expression.[66]
Some noncoding DNA sequences play structural roles in chromosomes. Telomeres and centromeres typically contain few genes but are important for the function and stability of chromosomes.[48][68] An abundant form of noncoding DNA in humans are pseudogenes, which are copies of genes that have been disabled by mutation.[69] These sequences are usually just molecular fossils, although they can occasionally serve as raw genetic material for the creation of new genes through the process of gene duplication and divergence.[70]
Transcription and translation
editA gene is a sequence of DNA that contains genetic information and can influence the phenotype of an organism. Within a gene, the sequence of bases along a DNA strand defines a messenger RNA sequence, which then defines one or more protein sequences. The relationship between the nucleotide sequences of genes and the amino-acid sequences of proteins is determined by the rules of translation, known collectively as the genetic code. The genetic code consists of three-letter 'words' called codons formed from a sequence of three nucleotides (e.g. ACT, CAG, TTT).
In transcription, the codons of a gene are copied into messenger RNA by RNA polymerase. This RNA copy is then decoded by a ribosome that reads the RNA sequence by base-pairing the messenger RNA to transfer RNA, which carries amino acids. Since there are 4 bases in 3-letter combinations, there are 64 possible codons (4 Various possible functions have been proposed for eDNA: it may be involved in horizontal gene transfer;[71] it may provide nutrients;[72] and it may act as a buffer to recruit or titrate ions or antibiotics.[73] Extracellular DNA acts as a functional extracellular matrix component in the biofilms of several bacterial species. It may act as a recognition factor to regulate the attachment and dispersal of specific cell types in the biofilm;[74] it may contribute to biofilm formation;[75] and it may contribute to the biofilm's physical strength and resistance to biological stress.[76] Cell-free fetal DNA is found in the blood of the mother, and can be sequenced to determine a great deal of information about the developing fetus.
Interactions with proteins
editAll the functions of DNA depend on interactions with proteins. These protein interactions can be non-specific, or the protein can bind specifically to a single DNA sequence. Enzymes can also bind to DNA and of these, the polymerases that copy the DNA base sequence in transcription and DNA replication are particularly important.
DNA-binding proteins
editLigases are particularly important in lagging strand DNA replication, as they join together the short segments of DNA produced at the replication fork into a complete copy of the DNA template. They are also used in DNA repair and genetic recombination.[77]
Topoisomerases and helicases
editTopoisomerases are enzymes with both nuclease and ligase activity. These proteins change the amount of supercoiling in DNA. Some of these enzymes work by cutting the DNA helix and allowing one section to rotate, thereby reducing its level of supercoiling; the enzyme then seals the DNA break.[78] Other types of these enzymes are capable of cutting one DNA helix and then passing a second strand of DNA through this break, before rejoining the helix.[79] Topoisomerases are required for many processes involving DNA, such as DNA replication and transcription.[29]
Helicases are proteins that are a type of molecular motor. They use the chemical energy in nucleoside triphosphates, predominantly adenosine triphosphate (ATP), to break hydrogen bonds between bases and unwind the DNA double helix into single strands.[80] These enzymes are essential for most processes where enzymes need to access the DNA bases.
Polymerases
editPolymerases are enzymes that synthesize polynucleotide chains from nucleoside triphosphates. The sequence of their products is created based on existing polynucleotide chains—which are called templates. These enzymes function by repeatedly adding a nucleotide to the 3′ hydroxyl group at the end of the growing polynucleotide chain. As a consequence, all polymerases work in a 5′ to 3′ direction.[81] In the active site of these enzymes, the incoming nucleoside triphosphate base-pairs to the template: this allows polymerases to accurately synthesize the complementary strand of their template. Polymerases are classified according to the type of template that they use.
In DNA replication, DNA-dependent DNA polymerases make copies of DNA polynucleotide chains. To preserve biological information, it is essential that the sequence of bases in each copy are precisely complementary to the sequence of bases in the template strand. Many DNA polymerases have a proofreading activity. Here, the polymerase recognizes the occasional mistakes in the synthesis reaction by the lack of base pairing between the mismatched nucleotides. If a mismatch is detected, a 3′ to 5′ exonuclease activity is activated and the incorrect base removed.[82] In most organisms, DNA polymerases function in a large complex called the replisome that contains multiple accessory subunits, such as the DNA clamp or helicases.[83]
RNA-dependent DNA polymerases are a specialized class of polymerases that copy the sequence of an RNA strand into DNA. They include reverse transcriptase, which is a viral enzyme involved in the infection of cells by retroviruses, and telomerase, which is required for the replication of telomeres.[47][84] Telomerase is an unusual polymerase because it contains its own RNA template as part of its structure.[48]
Transcription is carried out by a DNA-dependent RNA polymerase that copies the sequence of a DNA strand into RNA. To begin transcribing a gene, the RNA polymerase binds to a sequence of DNA called a promoter and separates the DNA strands. It then copies the gene sequence into a messenger RNA transcript until it reaches a region of DNA called the terminator, where it halts and detaches from the DNA. As with human DNA-dependent DNA polymerases, RNA polymerase II, the enzyme that transcribes most of the genes in the human genome, operates as part of a large protein complex with multiple regulatory and accessory subunits.[85]
Genetic recombination
edit[86] RNA may have acted as the central part of early cell metabolism as it can both transmit genetic information and carry out catalysis as part of ribozymes.[87] This ancient RNA world where nucleic acid would have been used for both catalysis and genetics may have influenced the evolution of the current genetic code based on four nucleotide bases. This would occur, since the number of different bases in such an organism is a trade-off between a small number of bases increasing replication accuracy and a large number of bases increasing the catalytic efficiency of ribozymes.[88] However, there is no direct evidence of ancient genetic systems, as recovery of DNA from most fossils is impossible because DNA survives in the environment for less than one million years, and slowly degrades into short fragments in solution.[89] Claims for older DNA have been made, most notably a report of the isolation of a viable bacterium from a salt crystal 250 million years old,[90] but these claims are controversial.[91][92]
Building blocks of DNA (adenine, guanine, and related organic molecules) may have been formed extraterrestrially in outer space.[93][94][95] Complex DNA and RNA organic compounds of life, including uracil, cytosine, and thymine, have also been formed in the laboratory under conditions mimicking those found in outer space, using starting chemicals, such as pyrimidine, found in meteorites. Pyrimidine, like polycyclic aromatic hydrocarbons (PAHs), the most carbon-rich chemical found in the universe, may have been formed in red giants or in interstellar cosmic dust and gas clouds.[96]
Uses in technology
editGenetic engineering
editMethods have been developed to purify DNA from organisms, such as phenol-chloroform extraction, and to manipulate it in the laboratory, such as restriction digests and the polymerase chain reaction. Modern biology and biochemistry make intensive use of these techniques in recombinant DNA technology. Recombinant DNA is a man-made DNA sequence that has been assembled from other DNA sequences. They can be transformed into organisms in the form of plasmids or in the appropriate format, by using a viral vector.[97] The genetically modified organisms produced can be used to produce products such as recombinant proteins, used in medical research,[98] or be grown in agriculture.[99][100]
DNA profiling
editForensic scientists can use DNA in blood, semen, skin, saliva or hair found at a crime scene to identify a matching DNA of an individual, such as a perpetrator. This process is formally termed DNA profiling, but may also be called "genetic fingerprinting". In DNA profiling, the lengths of variable sections of repetitive DNA, such as short tandem repeats and minisatellites, are compared between people. This method is usually an extremely reliable technique for identifying a matching DNA.[101] However, identification can be complicated if the scene is contaminated with DNA from several people.[102] DNA profiling was developed in 1984 by British geneticist Sir Alec Jeffreys,[103] and first used in forensic science to convict Colin Pitchfork in the 1988 Enderby murders case.[104]
The development of forensic science and the ability to now obtain genetic matching on minute samples of blood, skin, saliva, or hair has led to re-examining many cases. Evidence can now be uncovered that was scientifically impossible at the time of the original examination. Combined with the removal of the double jeopardy law in some places, this can allow cases to be reopened where prior trials have failed to produce sufficient evidence to convince a jury. People charged with serious crimes may be required to provide a sample of DNA for matching purposes. The most obvious defence to DNA matches obtained forensically is to claim that cross-contamination of evidence has occurred. This has resulted in meticulous strict handling procedures with new cases of serious crime. DNA profiling is also used successfully to positively identify victims of mass casualty incidents,[105] bodies or body parts in serious accidents, and individual victims in mass war graves, via matching to family members.
DNA profiling is also used in DNA paternity testing to determine if someone is the biological parent or grandparent of a child with the probability of parentage is typically 99.99% when the alleged parent is biologically related to the child. Normal DNA sequencing methods happen after birth but there are new methods to test paternity while a mother is still pregnant.[106]
DNA enzymes or catalytic DNA
editDeoxyribozymes, also called DNAzymes or catalytic DNA, are first discovered in 1994.[107] They are mostly single stranded DNA sequences isolated from a large pool of random DNA sequences through a combinatorial approach called in vitro selection or systematic evolution of ligands by exponential enrichment (SELEX). DNAzymes catalyze variety of chemical reactions including RNA-DNA cleavage, RNA-DNA ligation, amino acids phosphorylation-dephosphorylation, carbon-carbon bond formation, and etc. DNAzymes can enhance catalytic rate of chemical reactions up to 100,000,000,000-fold over the uncatalyzed reaction.[108] The most extensively studied class of DNAzymes is RNA-cleaving types which have been used to detect different metal ions and designing therapeutic agents. Several metal-specific DNAzymes have been reported including the GR-5 DNAzyme (lead-specific),[107] the CA1-3 DNAzymes (copper-specific),[109] the 39E DNAzyme (uranyl-specific) and the NaA43 DNAzyme (sodium-specific).[110] The NaA43 DNAzyme, which is reported to be more than 10,000-fold selective for sodium over other metal ions, was used to make a real-time sodium sensor in living cells.
Bioinformatics
editBioinformatics involves the development of techniques to store, data mine, search and manipulate biological data, including DNA nucleic acid sequence data. These have led to widely applied advances in computer science, especially string searching algorithms, machine learning, and database theory.[111] String searching or matching algorithms, which find an occurrence of a sequence of letters inside a larger sequence of letters, were developed to search for specific sequences of nucleotides.[112] The DNA sequence may be aligned with other DNA sequences to identify homologous sequences and locate the specific mutations that make them distinct. These techniques, especially multiple sequence alignment, are used in studying phylogenetic relationships and protein function.[113] Data sets representing entire genomes' worth of DNA sequences, such as those produced by the Human Genome Project, are difficult to use without the annotations that identify the locations of genes and regulatory elements on each chromosome. Regions of DNA sequence that have the characteristic patterns associated with protein- or RNA-coding genes can be identified by gene finding algorithms, which allow researchers to predict the presence of particular gene products and their possible functions in an organism even before they have been isolated experimentally.[114] Entire genomes may also be compared, which can shed light on the evolutionary history of particular organism and permit the examination of complex evolutionary events.
DNA nanotechnology
editDNA nanotechnology uses the unique molecular recognition properties of DNA and other nucleic acids to create self-assembling branched DNA complexes with useful properties.[115] DNA is thus used as a structural material rather than as a carrier of biological information. This has led to the creation of two-dimensional periodic lattices (both tile-based and using the DNA origami method) and three-dimensional structures in the shapes of polyhedra.[116] Nanomechanical devices and algorithmic self-assembly have also been demonstrated,[117] and these DNA structures have been used to template the arrangement of other molecules such as gold nanoparticles and streptavidin proteins.[118]
History and anthropology
editBecause DNA collects mutations over time, which are then inherited, it contains historical information, and, by comparing DNA sequences, geneticists can infer the evolutionary history of organisms, their phylogeny.[119] This field of phylogenetics is a powerful tool in evolutionary biology. If DNA sequences within a species are compared, population geneticists can learn the history of particular populations. This can be used in studies ranging from ecological genetics to anthropology; For example, DNA evidence is being used to try to identify the Ten Lost Tribes of Israel.[120][121]
Information storage
editIn a paper published in Nature in January 2013, scientists from the European Bioinformatics Institute and Agilent Technologies proposed a mechanism to use DNA's ability to code information as a means of digital data storage. The group was able to encode 739 kilobytes of data into DNA code, synthesize the actual DNA, then sequence the DNA and decode the information back to its original form, with a reported 100% accuracy. The encoded information consisted of text files and audio files. A prior experiment was published in August 2012. It was conducted by researchers at Harvard University, where the text of a 54,000-word book was encoded in DNA.[122][123]
Moreover, in living cells, the storage can be turned active by enzymes. Light-gated protein domains fused to DNA processing enzymes are suitable for that task in vitro.[124][125] Fluorescent exonucleases can transmit the output according to the nucleotide they have read.[126]
History of DNA research
editDNA was first isolated by the Swiss physician Friedrich Miescher who, in 1869, discovered a microscopic substance in the pus of discarded surgical bandages. As it resided in the nuclei of cells, he called it "nuclein".[127][128] In 1878, Albrecht Kossel isolated the non-protein component of "nuclein", nucleic acid, and later isolated its five primary nucleobases.[129][130] In 1919, Phoebus Levene identified the base, sugar, and phosphate nucleotide unit.[131] Levene suggested that DNA consisted of a string of nucleotide units linked together through the phosphate groups. Levene thought the chain was short and the bases repeated in a fixed order. In 1937, William Astbury produced the first X-ray diffraction patterns that showed that DNA had a regular structure.[132]
In 1927, Nikolai Koltsov proposed that inherited traits would be inherited via a "giant hereditary molecule" made up of "two mirror strands that would replicate in a semi-conservative fashion using each strand as a template".[133][134] In 1928, Frederick Griffith in his experiment discovered that traits of the "smooth" form of Pneumococcus could be transferred to the "rough" form of the same bacteria by mixing killed "smooth" bacteria with the live "rough" form.[135][136] This system provided the first clear suggestion that DNA carries genetic information—the Avery–MacLeod–McCarty experiment—when Oswald Avery, along with coworkers Colin MacLeod and Maclyn McCarty, identified DNA as the transforming principle in 1943.[137] DNA's role in heredity was confirmed in 1952 when Alfred Hershey and Martha Chase in the Hershey–Chase experiment showed that DNA is the genetic material of the T2 phage.[138]
In 1953, James Watson and Francis Crick suggested what is now accepted as the first correct double-helix model of DNA structure in the journal Nature.[34] Their double-helix, molecular model of DNA was then based on one X-ray diffraction image (labeled as "Photo 51")[139] taken by Rosalind Franklin and Raymond Gosling in May 1952, and the information that the DNA bases are paired.
Experimental evidence supporting the Watson and Crick model was published in a series of five articles in the same issue of Nature.[140] Of these, Franklin and Gosling's paper was the first publication of their own X-ray diffraction data and original analysis method that partly supported the Watson and Crick model;[32][141] this issue also contained an article on DNA structure by Maurice Wilkins and two of his colleagues, whose analysis and in vivo B-DNA X-ray patterns also supported the presence in vivo of the double-helical DNA configurations as proposed by Crick and Watson for their double-helix molecular model of DNA in the prior two pages of Nature.[33] In 1962, after Franklin's death, Watson, Crick, and Wilkins jointly received the Nobel Prize in Physiology or Medicine.[142] Nobel Prizes are awarded only to living recipients. A debate continues about who should receive credit for the discovery.[143]
In an influential presentation in 1957, Crick laid out the central dogma of molecular biology, which foretold the relationship between DNA, RNA, and proteins, and articulated the "adaptor hypothesis".[144] Final confirmation of the replication mechanism that was implied by the double-helical structure followed in 1958 through the Meselson–Stahl experiment.[145] Further work by Crick and coworkers showed that the genetic code was based on non-overlapping triplets of bases, called codons, allowing Har Gobind Khorana, Robert W. Holley, and Marshall Warren Nirenberg to decipher the genetic code.[146] These findings represent the birth of molecular biology.
See also
edit- Autosome
- Crystallography
- DNA-encoded chemical library
- DNA microarray
- DNA sequencing
- Macromolecule
- Genetic disorder
- Haplotype
- Comparison of nucleic acid simulation software
- Meiosis
- Mitochondrial DNA
- Nuclear DNA
- Nucleic acid double helix
- Nucleic acid notation
- Nucleic acid sequence
- Pangenesis
- Phosphoramidite
- Ribosomal DNA
- Southern blot
- X-ray scattering techniques
- Xeno nucleic acid
- RNA
- Deoxyribozyme
References
edit{{
- ^ Cite error: The named reference
Merriamwas invoked but never defined (see the help page). - ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2014). Molecular Biology of the Cell (6th ed.). Garland. p. Chapter 4: DNA, Chromosomes and Genomes. ISBN 9780815344322.
- ^ Purcell, Adam. "DNA". Basic Biology.
- ^ Mandelkern M, Elias JG, Eden D, Crothers DM (1981). "The dimensions of DNA in solution". J Mol Biol. 152 (1): 153–61. doi:10.1016/0022-2836(81)90099-1. PMID 7338906.
- ^ Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, et al. (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature. 441 (7091): 315–21. Bibcode:2006Natur.441..315G. doi:10.1038/nature04727. PMID 16710414.
- ^ Watson JD, Crick FH (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature. 171 (4356): 737–738. Bibcode:1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692. Retrieved 4 May 2009.
- ^ a b c Berg J., Tymoczko J. and Stryer L. (2002) Biochemistry. W. H. Freeman and Company ISBN 0-7167-4955-6
- ^ Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 3 January 2006.
- ^ a b Ghosh A, Bansal M (2003). "A glossary of DNA structures from A to Z". Acta Crystallogr D. 59 (4): 620–6. doi:10.1107/S0907444903003251. PMID 12657780.
- ^ Created from PDB 1D65
- ^ Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006). "Base-stacking and base-pairing contributions into thermal stability of the DNA double helix". Nucleic Acids Res. 34 (2): 564–74. doi:10.1093/nar/gkj454. PMC 1360284. PMID 16449200.
- ^ Burton E. Tropp - "Molecular Biology"- Jones and Barlett Learning, ISBN 978-0-7637-8663-2
- ^ "Watson-Crick Structure of DNA - 1953". Steven Carr. Memorial University of Newfoundland. Retrieved 13 July 2016.
- ^ Verma S, Eckstein F (1998). "Modified oligonucleotides: synthesis and strategy for users". Annu. Rev. Biochem. 67: 99–134. doi:10.1146/annurev.biochem.67.1.99. PMID 9759484.
- ^ Kiljunen S, Hakala K, Pinta E, Huttunen S, Pluta P, Gador A, Lönnberg H, Skurnik M (2005). "Yersiniophage phiR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine". Microbiology. 151 (12): 4093–4102. doi:10.1099/mic.0.28265-0. PMID 16339954.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Uchiyama J, Takemura-Uchiyama I, Sakaguchi Y, Gamoh K, Kato SI, Daibata M, Ujihara T, Misawa N, Matsuzaki S (March 2014). "Intragenus generalized transduction in Staphylococcus spp. by a novel giant phage". ISME J. 8: 1949–1952. doi:10.1038/ismej.2014.29.
- ^ Simpson L (1998). "A base called J". Proc Natl Acad Sci USA. 95 (5): 2037–2038. Bibcode:1998PNAS...95.2037S. doi:10.1073/pnas.95.5.2037. PMC 33841. PMID 9482833.
- ^ Borst P, Sabatini R (2008). "Base J: discovery, biosynthesis, and possible functions". Annual Review of Microbiology. 62: 235–51. doi:10.1146/annurev.micro.62.081307.162750. PMID 18729733.
- ^ Cross M, Kieft R, Sabatini R, Wilm M, de Kort M, van der Marel GA, van Boom JH, van Leeuwen F, Borst P (1999). "The modified base J is the target for a novel DNA-binding protein in kinetoplastid protozoans". The EMBO Journal. 18 (22): 6573–6581. doi:10.1093/emboj/18.22.6573. PMC 1171720. PMID 10562569.
- ^ DiPaolo C, Kieft R, Cross M, Sabatini R (2005). "Regulation of trypanosome DNA glycosylation by a SWI2/SNF2-like protein". Mol Cell. 17 (3): 441–451. doi:10.1016/j.molcel.2004.12.022. PMID 15694344.
- ^ Vainio S, Genest PA, ter Riet B, van Luenen H, Borst P (2009). "Evidence that J-binding protein 2 is a thymidine hydroxylase catalyzing the first step in the biosynthesis of DNA base J". Molecular and biochemical parasitology. 164 (2): 157–61. doi:10.1016/j.molbiopara.2008.12.001. PMID 19114062.
- ^ Iyer LM, Tahiliani M, Rao A, Aravind L (2009). "Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids". Cell Cycle. 8 (11): 1698–1710. doi:10.4161/cc.8.11.8580. PMC 2995806. PMID 19411852.
- ^ van Luenen HG, Farris C, Jan S, Genest PA, Tripathi P, Velds A, Kerkhoven RM, Nieuwland M, Haydock A, Ramasamy G, Vainio S, Heidebrecht T, Perrakis A, Pagie L, van Steensel B, Myler PJ, Borst P (2012). "Leishmania". Cell. 150 (5): 909–921. doi:10.1016/j.cell.2012.07.030. PMC 3684241. PMID 22939620.
- ^ Hazelbaker DZ, Buratowski S (2012). "Transcription: base J blocks the way". Curr Biol. 22 (22): R960–2. doi:10.1016/j.cub.2012.10.010. PMC 3648658. PMID 23174300.
- ^ Wing R, Drew H, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE (1980). "Crystal structure analysis of a complete turn of B-DNA". Nature. 287 (5784): 755–8. Bibcode:1980Natur.287..755W. doi:10.1038/287755a0. PMID 7432492.
- ^ Pabo CO, Sauer RT (1984). "Protein-DNA recognition". Annu Rev Biochem. 53: 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ^ Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub HE (2000). "Mechanical stability of single DNA molecules". Biophys J. 78 (4): 1997–2007. Bibcode:2000BpJ....78.1997C. doi:10.1016/S0006-3495(00)76747-6. PMC 1300792. PMID 10733978.
- ^ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walters, Peter (2002). Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1. OCLC 145080076.
- ^ a b Wang JC (2002). "Cellular roles of DNA topoisomerases: a molecular perspective". Nature Reviews Molecular Cell Biology. 3 (6): 430–40. doi:10.1038/nrm831. PMID 12042765.
- ^ Basu HS, Feuerstein BG, Zarling DA, Shafer RH, Marton LJ (1988). "Recognition of Z-RNA and Z-DNA determinants by polyamines in solution: experimental and theoretical studies". J Biomol Struct Dyn. 6 (2): 299–309. doi:10.1080/07391102.1988.10507714. PMID 2482766.
- ^ Franklin RE, Gosling RG (6 March 1953). "The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content" (PDF). Acta Crystallogr. 6 (8–9): 673–7. doi:10.1107/S0365110X53001939.
Franklin RE, Gosling RG (1953). "The structure of sodium thymonucleate fibres. II. The cylindrically symmetrical Patterson function". Acta Crystallogr. 6 (8–9): 678–85. doi:10.1107/S0365110X53001940. - ^ a b Franklin RE, Gosling RG (1953). "Molecular Configuration in Sodium Thymonucleate. Franklin R. and Gosling R.G" (PDF). Nature. 171 (4356): 740–1. Bibcode:1953Natur.171..740F. doi:10.1038/171740a0. PMID 13054694.
- ^ a b Wilkins MH, Stokes AR, Wilson HR (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature. 171 (4356): 738–740. Bibcode:1953Natur.171..738W. doi:10.1038/171738a0. PMID 13054693.
- ^ a b Watson JD, Crick FH (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature. 171 (4356): 737–738. Bibcode:1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692.
- ^ Leslie AG, Arnott S, Chandrasekaran R, Ratliff RL (1980). "Polymorphism of DNA double helices". J. Mol. Biol. 143 (1): 49–72. doi:10.1016/0022-2836(80)90124-2. PMID 7441761.
- ^ Baianu, I.C. (1980). "Structural Order and Partial Disorder in Biological systems". Bull. Math. Biol. 42 (4): 137–141. doi:10.1007/BF02462372. http://cogprints.org/3822/
- ^ Hosemann R., Bagchi R.N., Direct analysis of diffraction by matter, North-Holland Publs., Amsterdam – New York, 1962.
- ^ Baianu, I.C. (1978). "X-ray scattering by partially disordered membrane systems". Acta Crystallogr A. 34 (5): 751–753. Bibcode:1978AcCrA..34..751B. doi:10.1107/S0567739478001540.
- ^ Wahl MC, Sundaralingam M (1997). "Crystal structures of A-DNA duplexes". Biopolymers. 44 (1): 45–63. doi:10.1002/(SICI)1097-0282(1997)44:1<45::AID-BIP4>3.0.CO;2-#. PMID 9097733.
- ^ Lu XJ, Shakked Z, Olson WK (2000). "A-form conformational motifs in ligand-bound DNA structures". J. Mol. Biol. 300 (4): 819–40. doi:10.1006/jmbi.2000.3690. PMID 10891271.
- ^ Rothenburg S, Koch-Nolte F, Haag F (2001). "DNA methylation and Z-DNA formation as mediators of quantitative differences in the expression of alleles". Immunol Rev. 184: 286–98. doi:10.1034/j.1600-065x.2001.1840125.x. PMID 12086319.
- ^ Oh DB, Kim YG, Rich A (2002). "Z-DNA-binding proteins can act as potent effectors of gene expression in vivo". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16666–71. Bibcode:2002PNAS...9916666O. doi:10.1073/pnas.262672699. PMC 139201. PMID 12486233.
- ^ a b Palmer, Jason (2 December 2010). "Arsenic-loving bacteria may help in hunt for alien life". BBC News. Retrieved 2 December 2010.
- ^ a b Bortman, Henry (2 December 2010). "Arsenic-Eating Bacteria Opens New Possibilities for Alien Life". Space.com. Retrieved 2 December 2010.
- ^ Katsnelson, Alla (2 December 2010). "Arsenic-eating microbe may redefine chemistry of life". Nature News. doi:10.1038/news.2010.645.
- ^ Cressey, Daniel (3 October 2012). "'Arsenic-life' Bacterium Prefers Phosphorus after all". Nature News. doi:10.1038/nature.2012.11520.
- ^ a b Greider CW, Blackburn EH (1985). "Identification of a specific telomere terminal transferase activity in Tetrahymena extracts". Cell. 43 (2 Pt 1): 405–13. doi:10.1016/0092-8674(85)90170-9. PMID 3907856.
- ^ a b c Nugent CI, Lundblad V (1998). "The telomerase reverse transcriptase: components and regulation". Genes Dev. 12 (8): 1073–85. doi:10.1101/gad.12.8.1073. PMID 9553037.
- ^ Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW (1997). "Normal human chromosomes have long G-rich telomeric overhangs at one end". Genes Dev. 11 (21): 2801–9. doi:10.1101/gad.11.21.2801. PMC 316649. PMID 9353250.
- ^ Created from
- ^ a b Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S (2006). "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Res. 34 (19): 5402–15. doi:10.1093/nar/gkl655. PMC 1636468. PMID 17012276.
- ^ Parkinson GN, Lee MP, Neidle S (2002). "Crystal structure of parallel quadruplexes from human telomeric DNA". Nature. 417 (6891): 876–80. Bibcode:2002Natur.417..876P. doi:10.1038/nature755. PMID 12050675.
- ^ Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999). "Mammalian telomeres end in a large duplex loop". Cell. 97 (4): 503–14. doi:10.1016/S0092-8674(00)80760-6. PMID 10338214.
- ^ Alberts, B; Johnson A, Lewis J; et al. (2002). "The Preventable Causes of Cancer". Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-4072-9.
A certain irreducible background incidence of cancer is to be expected regardless of circumstances: mutations can never be absolutely avoided, because they are an inescapable consequence of fundamental limitations on the accuracy of DNA replication, as discussed in Chapter 5. If a human could live long enough, it is inevitable that at least one of his or her cells would eventually accumulate a set of mutations sufficient for cancer to develop.
- ^ Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak K (2008). Cancer and aging as consequences of un-repaired DNA damage. In: New Research on DNA Damages (Editors: Honoka Kimura and Aoi Suzuki) Nova Science Publishers, Inc., New York, Chapter 1, pp. 1–47. open access, but read only https://www.novapublishers.com/catalog/product_info.php?products_id=43247 ISBN 978-1604565812
- ^ Hoeijmakers JH (October 2009). "DNA damage, aging, and cancer". N. Engl. J. Med. 361 (15): 1475–85. doi:10.1056/NEJMra0804615. PMID 19812404.
- ^ Freitas AA, de Magalhães JP (2011). "A review and appraisal of the DNA damage theory of ageing". Mutat. Res. 728 (1–2): 12–22. doi:10.1016/j.mrrev.2011.05.001. PMID 21600302.
- ^ Ferguson LR, Denny WA (1991). "The genetic toxicology of acridines". Mutat Res. 258 (2): 123–60. doi:10.1016/0165-1110(91)90006-H. PMID 1881402.
- ^ Stephens TD, Bunde CJ, Fillmore BJ (2000). "Mechanism of action in thalidomide teratogenesis". Biochem Pharmacol. 59 (12): 1489–99. doi:10.1016/S0006-2952(99)00388-3. PMID 10799645.
- ^ Jeffrey AM (1985). "DNA modification by chemical carcinogens". Pharmacol Ther. 28 (2): 237–72. doi:10.1016/0163-7258(85)90013-0. PMID 3936066.
- ^ Braña MF, Cacho M, Gradillas A, de Pascual-Teresa B, Ramos A (2001). "Intercalators as anticancer drugs". Curr Pharm Des. 7 (17): 1745–80. doi:10.2174/1381612013397113. PMID 11562309.
- ^ Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (2001). "The sequence of the human genome". Science. 291 (5507): 1304–51. Bibcode:2001Sci...291.1304V. doi:10.1126/science.1058040. PMID 11181995.
- ^ Thanbichler M, Wang SC, Shapiro L (2005). "The bacterial nucleoid: a highly organized and dynamic structure". J Cell Biochem. 96 (3): 506–21. doi:10.1002/jcb.20519. PMID 15988757.
- ^ Wolfsberg TG, McEntyre J, Schuler GD (2001). "Guide to the draft human genome". Nature. 409 (6822): 824–6. Bibcode:2001Natur.409..824W. doi:10.1038/35057000. PMID 11236998.
- ^ Gregory TR (2005). "The C-value enigma in plants and animals: a review of parallels and an appeal for partnership". Annals of Botany. 95 (1): 133–46. doi:10.1093/aob/mci009. PMID 15596463.
- ^ Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, et al. (2007). "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Nature. 447 (7146): 799–816. Bibcode:2007Natur.447..799B. doi:10.1038/nature05874. PMC 2212820. PMID 17571346.
- ^ Created from PDB 1MSW
- ^ Pidoux AL, Allshire RC (2005). "The role of heterochromatin in centromere function". Philosophical Transactions of the Royal Society B. 360 (1455): 569–79. doi:10.1098/rstb.2004.1611. PMC 1569473. PMID 15905142.
- ^ Harrison PM, Hegyi H, Balasubramanian S, Luscombe NM, Bertone P, Echols N, Johnson T, Gerstein M (2002). "Molecular Fossils in the Human Genome: Identification and Analysis of the Pseudogenes in Chromosomes 21 and 22". Genome Res. 12 (2): 272–80. doi:10.1101/gr.207102. PMC 155275. PMID 11827946.
- ^ Harrison PM, Gerstein M (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol. 318 (5): 1155–74. doi:10.1016/S0022-2836(02)00109-2. PMID 12083509.
- ^ Vlassov, V. V.; Laktionov, P. P.; Rykova, E. Y. (2007). "Extracellular nucleic acids". BioEssays. 29: 654–667. doi:10.1002/bies.20604.
- ^ Finkel, S. E.; Kolter, R. (2001). "DNA as a nutrient: novel role for bacterial competence gene homologs". J. Bacteriol. 183: 6288–6293. doi:10.1128/JB.183.21.6288-6293.2001.
- ^ Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. (2008). "Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms". PLoS Pathog. 4: e1000213. doi:10.1371/journal.ppat.1000213.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Berne, C.; Kysela, D. T.; Brun, Y. V. (2010). "A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm". Mol. Microbiol. 77: 815–829. doi:10.1111/j.1365-2958.2010.07267.x.
- ^ Whitchurch, C. B.; Tolker-Nielsen, T.; Ragas, P. C.; Mattick, J. S. (2002). "Extracellular DNA required for bacterial biofilm formation" (PDF). Science. 295: 1487. doi:10.1126/science.295.5559.1487.
- ^ Hu, W.; Li, L.; Sharma, S.; Wang, J.; McHardy, I.; Lux, R.; Yang, Z.; He, X.; Gimzewski, J. K.; Li, Y.; Shi, W. (2012). "DNA Builds and Strengthens the Extracellular Matrix in Myxococcus xanthus Biofilms by Interacting with Exopolysaccharides". PLoS ONE. 7 (12): e51905. Bibcode:2012PLoSO...751905H. doi:10.1371/journal.pone.0051905.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Doherty AJ, Suh SW (2000). "Structural and mechanistic conservation in DNA ligases". Nucleic Acids Res. 28 (21): 4051–8. doi:10.1093/nar/28.21.4051. PMC 113121. PMID 11058099.
- ^ Champoux JJ (2001). "DNA topoisomerases: structure, function, and mechanism". Annu Rev Biochem. 70: 369–413. doi:10.1146/annurev.biochem.70.1.369. PMID 11395412.
- ^ Schoeffler AJ, Berger JM (2005). "Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism". Biochem Soc Trans. 33 (Pt 6): 1465–70. doi:10.1042/BST20051465. PMID 16246147.
- ^ Tuteja N, Tuteja R (2004). "Unraveling DNA helicases. Motif, structure, mechanism and function". Eur J Biochem. 271 (10): 1849–63. doi:10.1111/j.1432-1033.2004.04094.x. PMID 15128295.
- ^ Joyce CM, Steitz TA (1995). "Polymerase structures and function: variations on a theme?". J Bacteriol. 177 (22): 6321–9. PMC 177480. PMID 7592405.
- ^ Hubscher U, Maga G, Spadari S (2002). "Eukaryotic DNA polymerases". Annu Rev Biochem. 71: 133–63. doi:10.1146/annurev.biochem.71.090501.150041. PMID 12045093.
- ^ Johnson A, O'Donnell M (2005). "Cellular DNA replicases: components and dynamics at the replication fork". Annu Rev Biochem. 74: 283–315. doi:10.1146/annurev.biochem.73.011303.073859. PMID 15952889.
- ^ Tarrago-Litvak L, Andréola ML, Nevinsky GA, Sarih-Cottin L, Litvak S (1 May 1994). "The reverse transcriptase of HIV-1: from enzymology to therapeutic intervention". FASEB J. 8 (8): 497–503. PMID 7514143.
- ^ Martinez E (2002). "Multi-protein complexes in eukaryotic gene transcription". Plant Mol Biol. 50 (6): 925–47. doi:10.1023/A:1021258713850. PMID 12516863.
- ^ Orgel LE (2004). "Prebiotic chemistry and the origin of the RNA world". Crit Rev Biochem Mol Biol. 39 (2): 99–123. doi:10.1080/10409230490460765. PMID 15217990.
- ^ Davenport RJ (2001). "Ribozymes. Making copies in the RNA world". Science. 292 (5520): 1278. doi:10.1126/science.292.5520.1278a. PMID 11360970.
- ^ Szathmáry E (1992). "What is the optimum size for the genetic alphabet?". Proc Natl Acad Sci USA. 89 (7): 2614–8. Bibcode:1992PNAS...89.2614S. doi:10.1073/pnas.89.7.2614. PMC 48712. PMID 1372984.
- ^ Lindahl T (1993). "Instability and decay of the primary structure of DNA". Nature. 362 (6422): 709–15. Bibcode:1993Natur.362..709L. doi:10.1038/362709a0. PMID 8469282.
- ^ Vreeland RH, Rosenzweig WD, Powers DW (2000). "Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal". Nature. 407 (6806): 897–900. doi:10.1038/35038060. PMID 11057666.
- ^ Hebsgaard MB, Phillips MJ, Willerslev E (2005). "Geologically ancient DNA: fact or artefact?". Trends Microbiol. 13 (5): 212–20. doi:10.1016/j.tim.2005.03.010. PMID 15866038.
- ^ Nickle DC, Learn GH, Rain MW, Mullins JI, Mittler JE (2002). "Curiously modern DNA for a "250 million-year-old" bacterium". J Mol Evol. 54 (1): 134–7. doi:10.1007/s00239-001-0025-x. PMID 11734907.
- ^ Callahan MP, Smith KE, Cleaves HJ, Ruzicka J, Stern JC, Glavin DP, House CH, Dworkin JP (August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". Proc. Natl. Acad. Sci. U.S.A. 108 (34): 13995–8. Bibcode:2011PNAS..10813995C. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
- ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 10 August 2011.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 9 August 2011.
- ^ Marlaire, Ruth (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". NASA. Retrieved 5 March 2015.
- ^ Goff SP, Berg P (1976). "Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells". Cell. 9 (4 PT 2): 695–705. doi:10.1016/0092-8674(76)90133-1. PMID 189942.
- ^ Houdebine LM (2007). "Transgenic animal models in biomedical research". Methods Mol Biol. 360: 163–202. doi:10.1385/1-59745-165-7:163. ISBN 1-59745-165-7. PMID 17172731.
- ^ Daniell H, Dhingra A (2002). "Multigene engineering: dawn of an exciting new era in biotechnology". Current Opinion in Biotechnology. 13 (2): 136–41. doi:10.1016/S0958-1669(02)00297-5. PMC 3481857. PMID 11950565.
- ^ Job D (2002). "Plant biotechnology in agriculture". Biochimie. 84 (11): 1105–10. doi:10.1016/S0300-9084(02)00013-5. PMID 12595138.
- ^ Collins A, Morton NE (1994). "Likelihood ratios for DNA identification". Proc Natl Acad Sci USA. 91 (13): 6007–11. Bibcode:1994PNAS...91.6007C. doi:10.1073/pnas.91.13.6007. PMC 44126. PMID 8016106.
- ^ Weir BS, Triggs CM, Starling L, Stowell LI, Walsh KA, Buckleton J (1997). "Interpreting DNA mixtures". J Forensic Sci. 42 (2): 213–22. PMID 9068179.
- ^ Jeffreys AJ, Wilson V, Thein SL (1985). "Individual-specific 'fingerprints' of human DNA". Nature. 316 (6023): 76–9. Bibcode:1985Natur.316...76J. doi:10.1038/316076a0. PMID 2989708.
- ^ Colin Pitchfork — first murder conviction on DNA evidence also clears the prime suspect Forensic Science Service Accessed 23 December 2006
- ^ "DNA Identification in Mass Fatality Incidents". National Institute of Justice. September 2006.
- ^ "Paternity Blood Tests That Work Early in a Pregnancy" New York Times June 20, 2012
- ^ a b Breaker, Ronald R.; Joyce, Gerald F. (12 January 1994). "A DNA enzyme that cleaves RNA". Chemistry & Biology. 1 (4): 223–229. doi:10.1016/1074-5521(94)90014-0. ISSN 1074-5521. PMID 9383394.
- ^ Chandra, Madhavaiah; Sachdeva, Amit; Silverman, Scott K. "DNA-catalyzed sequence-specific hydrolysis of DNA". Nature Chemical Biology. 5 (10): 718–720. doi:10.1038/nchembio.201. PMC 2746877. PMID 19684594.
- ^ Carmi, Nir; Shultz, Lisa A.; Breaker, Ronald R. (12 January 1996). "In vitro selection of self-cleaving DNAs". Chemistry & Biology. 3 (12): 1039–1046. doi:10.1016/S1074-5521(96)90170-2. ISSN 1074-5521. PMID 9000012.
- ^ Torabi, Seyed-Fakhreddin; Wu, Peiwen; McGhee, Claire E.; Chen, Lu; Hwang, Kevin; Zheng, Nan; Cheng, Jianjun; Lu, Yi (12 May 2015). "In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing". Proceedings of the National Academy of Sciences. 112 (19): 5903–5908. doi:10.1073/pnas.1420361112. ISSN 0027-8424. PMC 4434688. PMID 25918425.
- ^ Baldi, Pierre; Brunak, Soren (2001). Bioinformatics: The Machine Learning Approach. MIT Press. ISBN 978-0-262-02506-5. OCLC 45951728.
- ^ Gusfield, Dan. Algorithms on Strings, Trees, and Sequences: Computer Science and Computational Biology. Cambridge University Press, 15 January 1997. ISBN 978-0-521-58519-4.
- ^ Sjölander K (2004). "Phylogenomic inference of protein molecular function: advances and challenges". Bioinformatics. 20 (2): 170–9. doi:10.1093/bioinformatics/bth021. PMID 14734307.
- ^ Mount DM (2004). Bioinformatics: Sequence and Genome Analysis (2 ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 0-87969-712-1. OCLC 55106399.
- ^ Rothemund PW (2006). "Folding DNA to create nanoscale shapes and patterns". Nature. 440 (7082): 297–302. Bibcode:2006Natur.440..297R. doi:10.1038/nature04586. PMID 16541064.
- ^ Andersen ES, Dong M, Nielsen MM, Jahn K, Subramani R, Mamdouh W, Golas MM, Sander B, Stark H, Oliveira CL, Pedersen JS, Birkedal V, Besenbacher F, Gothelf KV, Kjems J (2009). "Self-assembly of a nanoscale DNA box with a controllable lid". Nature. 459 (7243): 73–6. Bibcode:2009Natur.459...73A. doi:10.1038/nature07971. PMID 19424153.
- ^ Ishitsuka Y, Ha T (2009). "DNA nanotechnology: a nanomachine goes live". Nat Nanotechnol. 4 (5): 281–2. Bibcode:2009NatNa...4..281I. doi:10.1038/nnano.2009.101. PMID 19421208.
- ^ Aldaye FA, Palmer AL, Sleiman HF (2008). "Assembling materials with DNA as the guide". Science. 321 (5897): 1795–9. Bibcode:2008Sci...321.1795A. doi:10.1126/science.1154533. PMID 18818351.
- ^ Wray GA (2002). "Dating branches on the Tree of Life using DNA". Genome Biol. 3 (1): reviews0001.1–reviews0001.7. doi:10.1046/j.1525-142X.1999.99010.x. PMC 150454. PMID 11806830.
{{cite journal}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ Lost Tribes of Israel, Nova, PBS airdate: 22 February 2000. Transcript available from PBS.org. Retrieved 4 March 2006.
- ^ Kleiman, Yaakov. "The Cohanim/DNA Connection: The fascinating story of how DNA studies confirm an ancient biblical tradition". aish.com (13 January 2000). Retrieved 4 March 2006.
- ^ Goldman N, Bertone P, Chen S, Dessimoz C, LeProust EM, Sipos B, Birney E (23 January 2013). "Towards practical, high-capacity, low-maintenance information storage in synthesized DNA". Nature. 494 (7435): 77–80. Bibcode:2013Natur.494...77G. doi:10.1038/nature11875. PMC 3672958. PMID 23354052.
- ^ Naik, Gautam (24 January 2013). "Storing Digital Data in DNA". Wall Street Journal. Retrieved 24 January 2013.
- ^ Comment by Dandekar, T., Lopez, D., Schaack, D. (2013) http://www.nature.com/nature/journal/v494/n7435/abs/nature11875.html#comment-57415
- ^ Emerging Technology Final, Dandekar T., Lopez, D., Programmable bacterial membranes with active DNA storage; presentation for the University of Würzburg for the Royal Society for Chemistry, London, 2016-06-29
- ^ Patent "Molecular highly integrated data storage via active control DNA", DE102013004584 A1, https://www.google.com/patents/DE102013004584A1"
- ^ Miescher, Friedrich (1871) "Ueber die chemische Zusammensetzung der Eiterzellen" (On the chemical composition of pus cells), Medicinisch-chemische Untersuchungen, 4 : 441–460. From p. 456: "Ich habe mich daher später mit meinen Versuchen an die ganzen Kerne gehalten, die Trennung der Körper, die ich einstweilen ohne weiteres Präjudiz als lösliches und unlösliches Nuclein bezeichnen will, einem günstigeren Material überlassend." (Therefore, in my experiments I subsequently limited myself to the whole nucleus, leaving to a more favorable material the separation of the substances, that for the present, without further prejudice, I will designate as soluble and insoluble nuclear material ("Nuclein").)
- ^ Dahm R (2008). "Discovering DNA: Friedrich Miescher and the early years of nucleic acid research". Hum. Genet. 122 (6): 565–81. doi:10.1007/s00439-007-0433-0. PMID 17901982.
- ^ See:
- Albrect Kossel (1879) "Ueber Nucleïn der Hefe" (On nuclein in yeast) Zeitschrift für physiologische Chemie, 3 : 284-291.
- Albrect Kossel (1880) "Ueber Nucleïn der Hefe II" (On nuclein in yeast, Part 2) Zeitschrift für physiologische Chemie, 4 : 290-295.
- Albrect Kossel (1881) "Ueber die Verbreitung des Hypoxanthins im Thier- und Pflanzenreich" (On the distribution of hypoxanthins in the animal and plant kingdoms) Zeitschrift für physiologische Chemie, 5 : 267-271.
- Albrect Kossel, Untersuchungen über die Nucleine und ihre Spaltungsprodukte [Investigations into nuclein and its cleavage products] (Strassburg, Germany: K.J. Trübne, 1881), 19 pages.
- Albrect Kossel (1882) "Ueber Xanthin und Hypoxanthin" (On xanthin and hypoxanthin), Zeitschrift für physiologische Chemie, 6 : 422-431.
- Albrect Kossel (1883) "Zur Chemie des Zellkerns" (On the chemistry of the cell nucleus), Zeitschrift für physiologische Chemie, 7 : 7-22.
- Albrect Kossel (1886) "Weitere Beiträge zur Chemie des Zellkerns" (Further contributions to the chemistry of the cell nucleus), Zeitschrift für Physiologische Chemie, 10 : 248-264. Available on-line at: Max Planck Institute for the History of Science, Berlin, Germany. On p. 264, Kossel remarked presciently: "Der Erforschung der quantitativen Verhältnisse der vier stickstoffreichen Basen, der Abhängigkeit ihrer Menge von den physiologischen Zuständen der Zelle, verspricht wichtige Aufschlüsse über die elementaren physiologisch-chemischen Vorgänge." (The study of the quantitative relations of the four nitrogenous bases — [and] of the dependence of their quantity on the physiological states of the cell — promises important insights into the fundamental physiological-chemical processes.)
- ^ Jones ME (September 1953). "Albrecht Kossel, A Biographical Sketch". Yale Journal of Biology and Medicine. 26 (1). National Center for Biotechnology Information: 80–97. PMC 2599350. PMID 13103145.
{{cite journal}}: CS1 maint: year (link) - ^ Levene P (1 December 1919). "The structure of yeast nucleic acid". J Biol Chem. 40 (2): 415–24.
- ^ See:
- W. T. Astbury and Florence O. Bell (1938) "Some recent developments in the X-ray study of proteins and related structures," Cold Spring Harbor Symposia on Quantitative Biology, 6 : 109-121. Available on-line at: University of Leeds.
- Astbury, W. T., (1947) "X-ray studies of nucleic acids," Symposia of the Society for Experimental Biology, 1 : 66-76. Available on-line at: Oregon State University.
- ^ Koltsov proposed that a cell's genetic information was encoded in a long chain of amino acids. See:
- Н. К. Кольцов, "Физико-химические основы морфологии" (The physical-chemical basis of morphology) -- speech given at the 3rd All-Union Meeting of Zoologist, Anatomists, and Histologists at Leningrad, U.S.S.R., December 12, 1927.
- Reprinted in: Успехи экспериментальной биологии (Advances in Experimental Biology), series B, 7 (1) : ?-? (1928).
- Reprinted in German as: Nikolaj K. Koltzoff (1928) "Physikalisch-chemische Grundlagen der Morphologie" (The physical-chemical basis of morphology), Biologisches Zentralblatt, 48 (6) : 345-369.
- In 1934, Koltsov contended that the proteins that contain a cell's genetic information replicate. See: N. K. Koltzoff (October 5, 1934) "The structure of the chromosomes in the salivary glands of Drosophila," Science, 80 (2075) : 312-313. From page 313: "I think that the size of the chromosomes in the salivary glands [of Drosophila] is determined through the multiplication of genonemes. By this term I designate the axial thread of the chromosome, in which the geneticists locate the linear combination of genes; … In the normal chromosome there is usually only one genoneme; before cell-division this genoneme has become divided into two strands."
- ^ Soyfer VN (2001). "The consequences of political dictatorship for Russian science". Nature Reviews Genetics. 2 (9): 723–729. doi:10.1038/35088598. PMID 11533721.
- ^ Griffith F (January 1928). "The significance of pneumococcal types". The Journal of Hygiene (London). 27 (2): 113–59. doi:10.1017/S0022172400031879. PMC 2167760. PMID 20474956.
- ^ Lorenz MG, Wackernagel W (1994). "Bacterial gene transfer by natural genetic transformation in the environment". Microbiol. Rev. 58 (3): 563–602. PMC 372978. PMID 7968924.
- ^ Avery OT, Macleod CM, McCarty M (1944). "Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii". J Exp Med. 79 (2): 137–158. doi:10.1084/jem.79.2.137. PMC 2135445. PMID 19871359.
- ^ Hershey AD, Chase M (1952). "Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage". J Gen Physiol. 36 (1): 39–56. doi:10.1085/jgp.36.1.39. PMC 2147348. PMID 12981234.
- ^ The B-DNA X-ray pattern on the right of this linked image was obtained by Rosalind Franklin and Raymond Gosling in May 1952 at high hydration levels of DNA and it has been labeled as "Photo 51"
- ^ Nature Archives Double Helix of DNA: 50 Years
- ^ "Original X-ray diffraction image". Osulibrary.oregonstate.edu. Retrieved 6 February 2011.
- ^ The Nobel Prize in Physiology or Medicine 1962 Nobelprize .org Accessed 22 December 06
- ^ Maddox B (23 January 2003). "The double helix and the 'wronged heroine'" (PDF). Nature. 421 (6921): 407–408. Bibcode:2003Natur.421..407M. doi:10.1038/nature01399. PMID 12540909.
- ^ Crick, F.H.C. On degenerate templates and the adaptor hypothesis (PDF). genome.wellcome.ac.uk (Lecture, 1955). Retrieved 22 December 2006.
- ^ Meselson M, Stahl FW (1958). "The replication of DNA in Escherichia coli". Proc Natl Acad Sci USA. 44 (7): 671–82. Bibcode:1958PNAS...44..671M. doi:10.1073/pnas.44.7.671. PMC 528642. PMID 16590258.
- ^ The Nobel Prize in Physiology or Medicine 1968 Nobelprize.org Accessed 22 December 06
Cite error: A list-defined reference named "NYT-20150718-rn" is not used in the content (see the help page).
Cite error: A list-defined reference named "AGCI-2015" is not used in the content (see the help page).
Cite error: A list-defined reference named "RusGenet" is not used in the content (see the help page).
Cite error: A list-defined reference named "Mashaghi" is not used in the content (see the help page).
Cite error: A list-defined reference named "Saenger" is not used in the content (see the help page).
Cite error: A list-defined reference named "SuperCoil" is not used in the content (see the help page).
Cite error: A list-defined reference named "breslauer" is not used in the content (see the help page).
Cite error: A list-defined reference named "hlmnn" is not used in the content (see the help page).
Cite error: A list-defined reference named "cheruku" is not used in the content (see the help page).
Cite error: A list-defined reference named "qmul" is not used in the content (see the help page).
Cite error: A list-defined reference named "httnhfr" is not used in the content (see the help page).
Cite error: A list-defined reference named "mnroe" is not used in the content (see the help page).
Cite error: A list-defined reference named "mklwsk" is not used in the content (see the help page).
Cite error: A list-defined reference named "Johnsholm" is not used in the content (see the help page).
Cite error: A list-defined reference named "lambhorv" is not used in the content (see the help page).
Cite error: A list-defined reference named "benlke" is not used in the content (see the help page).
Cite error: A list-defined reference named "seeNCman" is not used in the content (see the help page).
Cite error: A list-defined reference named "Rosen01" is not used in the content (see the help page).
Cite error: A list-defined reference named "klose" is not used in the content (see the help page).
Cite error: A list-defined reference named "bird01" is not used in the content (see the help page).
Cite error: A list-defined reference named "walshxu" is not used in the content (see the help page).
Cite error: A list-defined reference named "krcnis" is not used in the content (see the help page).
Cite error: A list-defined reference named "rrbw" is not used in the content (see the help page).
Cite error: A list-defined reference named "vliegen" is not used in the content (see the help page).
Cite error: A list-defined reference named "rcsborg01" is not used in the content (see the help page).
Cite error: A list-defined reference named "douki" is not used in the content (see the help page).
Cite error: A list-defined reference named "delatour" is not used in the content (see the help page).
Cite error: A list-defined reference named "beck01" is not used in the content (see the help page).
Cite error: A list-defined reference named "valerie" is not used in the content (see the help page).
Cite error: A list-defined reference named "Weinberg" is not used in the content (see the help page).
Cite error: A list-defined reference named "replica" is not used in the content (see the help page).
Cite error: A list-defined reference named "Tani_2010" is not used in the content (see the help page).
Cite error: A list-defined reference named "propro" is not used in the content (see the help page).
Cite error: A list-defined reference named "nucleoasso" is not used in the content (see the help page).
Cite error: A list-defined reference named "structnuc" is not used in the content (see the help page).
Cite error: A list-defined reference named "transhistone" is not used in the content (see the help page).
Cite error: A list-defined reference named "nucassem" is not used in the content (see the help page).
Cite error: A list-defined reference named "archbind" is not used in the content (see the help page).
Cite error: A list-defined reference named "assemnucstruct" is not used in the content (see the help page).
Cite error: A list-defined reference named "reppro" is not used in the content (see the help page).
Cite error: A list-defined reference named "rcsborg03" is not used in the content (see the help page).
Cite error: A list-defined reference named "mediatortrans" is not used in the content (see the help page).
Cite error: A list-defined reference named "bioconco" is not used in the content (see the help page).
Cite error: A list-defined reference named "globtransreg" is not used in the content (see the help page).
Cite error: A list-defined reference named "rcsborg04" is not used in the content (see the help page).
Cite error: A list-defined reference named "giorestric" is not used in the content (see the help page).
Cite error: A list-defined reference named "rcsborg05" is not used in the content (see the help page).
Cite error: A list-defined reference named "regmamcell" is not used in the content (see the help page).
Cite error: A list-defined reference named "lerchInte" is not used in the content (see the help page).
Cite error: A list-defined reference named "JeggoInsite" is not used in the content (see the help page).
Cite error: A list-defined reference named "mamradpro" is not used in the content (see the help page).
Cite error: A list-defined reference named "clarimech" is not used in the content (see the help page).
Cite error: A list-defined reference named "resolva" is not used in the content (see the help page).
Further reading
edit- Berry, Andrew; Watson, James. (2003). DNA: the secret of life. New York: Alfred A. Knopf. ISBN 0-375-41546-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Calladine, Chris R.; Drew, Horace R.; Luisi, Ben F.; Travers, Andrew A. (2003). Understanding DNA: the molecule & how it works. Amsterdam: Elsevier Academic Press. ISBN 0-12-155089-3.
- Dennis, Carina; Julie Clayton (2003). 50 years of DNA. Basingstoke: Palgrave Macmillan. ISBN 1-4039-1479-6.
- Judson, Horace F. 1979. The Eighth Day of Creation: Makers of the Revolution in Biology. Touchstone Books, ISBN 0-671-22540-5. 2nd edition: Cold Spring Harbor Laboratory Press, 1996 paperback: ISBN 0-87969-478-5.
- Olby, Robert C. (1994). The path to the double helix: the discovery of DNA. New York: Dover Publications. ISBN 0-486-68117-3., first published in October 1974 by MacMillan, with foreword by Francis Crick; the definitive DNA textbook, revised in 1994 with a 9-page postscript
- Micklas, David. 2003. DNA Science: A First Course. Cold Spring Harbor Press: ISBN 978-0-87969-636-8
- Ridley, Matt (2006). Francis Crick: discoverer of the genetic code. Ashland, OH: Eminent Lives, Atlas Books. ISBN 0-06-082333-X.
- Olby, Robert C. (2009). Francis Crick: A Biography. Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 0-87969-798-9.
- Rosenfeld, Israel. 2010. DNA: A Graphic Guide to the Molecule that Shook the World. Columbia University Press: ISBN 978-0-231-14271-7
- Schultz, Mark and Zander Cannon. 2009. The Stuff of Life: A Graphic Guide to Genetics and DNA. Hill and Wang: ISBN 0-8090-8947-5
- Stent, Gunther Siegmund; Watson, James. (1980). The Double Helix: A Personal Account of the Discovery of the Structure of DNA. New York: Norton. ISBN 0-393-95075-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Watson, James. 2004. DNA: The Secret of Life. Random House: ISBN 978-0-09-945184-6
- Wilkins, Maurice (2003). The third man of the double helix the autobiography of Maurice Wilkins. Cambridge, Eng: University Press. ISBN 0-19-860665-6.
External links
edit- DNA binding site prediction on protein
- DNA the Double Helix Game From the official Nobel Prize web site
- DNA under electron microscope
- Dolan DNA Learning Center
- Double Helix: 50 years of DNA, Nature
- Proteopedia DNA
- Proteopedia Forms_of_DNA
- ENCODE threads explorer ENCODE home page. Nature (journal)
- Double Helix 1953–2003 National Centre for Biotechnology Education
- Genetic Education Modules for Teachers—DNA from the Beginning Study Guide
- PDB Molecule of the Month DNA
- Rosalind Franklin's contributions to the study of DNA
- U.S. National DNA Day—watch videos and participate in real-time chat with top scientists
- Clue to chemistry of heredity found The New York Times June 1953. First American newspaper coverage of the discovery of the DNA structure
- Olby R (2003). "Quiet debut for the double helix". Nature. 421 (6921): 402–5. Bibcode:2003Natur.421..402O. doi:10.1038/nature01397. PMID 12540907.
- DNA from the Beginning Another DNA Learning Center site on DNA, genes, and heredity from Mendel to the human genome project.
- The Register of Francis Crick Personal Papers 1938 – 2007 at Mandeville Special Collections Library, University of California, San Diego
- Seven-page, handwritten letter that Crick sent to his 12-year-old son Michael in 1953 describing the structure of DNA. See Crick’s medal goes under the hammer, Nature, 5 April 2013.
- 3D map of DNA reveals hidden loops that allow genes to work together (11 December 2014), Science (Daily News)
Template:Featured article is only for Wikipedia:Featured articles.