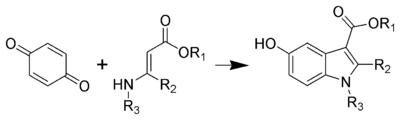
The Nenitzescu indole synthesis is a chemical reaction that forms 5-hydroxyindole derivatives from benzoquinone and β-aminocrotonic esters.
Mechanism
editThe mechanism of a Nenitzescu reaction consists of a conjugate addition, followed by a nucleophilic attack by the C–N pi bond, and then an elimination.
The reaction was first published by Nenitzescu in 1929,[1] and since then...
Related Reactions
editThere are several other
References
edit- Nenitzescu, C. D. Bull. Soc. Chim. Romania 1929, 11, 37.
- Allen, Jr., G. R. et al. J. Am. Chem. Soc. 1966, 88(11), 2536–2544. PMID 5941382
- Allen, Jr., G. R. Org. React. 1973, 20, 337.
- Pawlak, J. M. et al. J. Org. Chem. 1996, 61, 9055.
Category:Indole forming reactions Category:Carbon-heteroatom bond forming reactions
