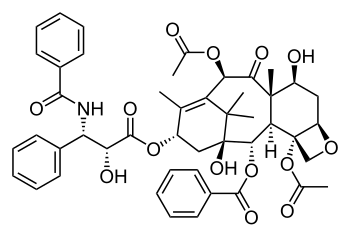
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | iv |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 6.5% (oral)[1] |
| Protein binding | 89 to 98% |
| Metabolism | Hepatic (CYP2C8 and CYP3A4) |
| Elimination half-life | 5.8 hours |
| Excretion | Fecal and urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C47H51NO14 |
| Molar mass | 853.906 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a U.S. National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol. When it was developed commercially by Bristol-Myers Squibb (BMS) the generic name was changed to paclitaxel and the BMS compound is sold under the trademark TAXOL. In this formulation, paclitaxel is dissolved in Cremophor EL and ethanol, as a delivery agent. A newer formulation, in which paclitaxel is bound to albumin, is sold under the trademark Abraxane.
Paclitaxel is now used to treat patients with lung, ovarian, breast cancer, head and neck cancer, and advanced forms of Kaposi's sarcoma. Paclitaxel is also used for the prevention of restenosis.
Paclitaxel stabilizes microtubules and as a result, interferes with the normal breakdown of microtubules during cell division. Together with docetaxel, it forms the drug category of the taxanes. It was the subject of a notable total synthesis by Robert A. Holton.
While offering substantial improvement in patient care, paclitaxel has been a relatively controversial drug. There was originally concern because of the environmental impact of its original sourcing, no longer used, from the Pacific yew. In addition, the assignment of rights, and even the name itself, to Bristol-Myers Squibb were the subject of public debate and Congressional hearings.
Nomenclature
editThe nomenclature for paclitaxel is structured on a tetracyclic 17-carbon (heptadecane) skeleton. There are a total of 11 stereocenters. The active stereoisomer is (-)-paclitaxel (shown here).
|
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-Diacetoxy-15-{[(2R,3S)-3- (benzoylamino)-2-hydroxy-3- phenylpropanoyl]oxy}-1,9- dihydroxy-10,14,17,17-tetramethyl -11-oxo-6-oxatetracyclo [11.3.1.0~3,10~.0~4,7~] heptadec-13-en-2-yl rel-benzoate |
History
editThe plant screening program, isolation, and preclinical trials
editIn 1955, the National Cancer Institute (NCI) set up the Cancer Chemotherapy National Service Center (CCNSC) to act as a public screening center for anticancer activity in compounds submitted by external institutions and companies.[2] Although the majority of compounds screened were of synthetic origin, one chemist, Jonathan Hartwell, who was employed there from 1958 onwards, had had experience with natural product derived compounds, and began a plant screening operation.[3] After some years of informal arrangements, in July 1960, the NCI commissioned USDA botanists to collect samples from about 1000 plant species per year.[4] On 21 August 1962, one of those botanists, Arthur S. Barclay, collected bark from a single Pacific yew tree, Taxus brevifolia, in a forest north of the town of Packwood, Washington as part of a four month trip to collect material from over 200 different species.[5] The material was then processed by a number of specialist CCNSC subcontractors, and one of the Taxus samples was found to be cytotoxic in a cellular assay on 22 May 1964.[5]
Accordingly, in late 1964 or early 1965, the fractionation and isolation laboratory run by Monroe E. Wall in Research Triangle Park, North Carolina, began work on fresh Taxus samples, isolating the active ingredient in September 1966 and announcing their findings at an April 1967 American Chemical Society meeting in Miami Beach.[6] They named the pure compound taxol in June 1967.[7] Wall and his colleague Wani published their results, including the chemical structure, in 1971.[8]
The NCI continued to commission work to collect more Taxus bark and to isolate increasing quantities of taxol. By 1969, 28 kg of crude extract had been isolated from almost 1,200 kg of bark, although this ultimately yielded only 10g of pure material,[9] but for several years, no use was made of the compound by the NCI. In 1975, it was shown to be active in another in vitro system ; two years later a new department head reviewed the data and finally recommended taxol be moved on to the next stage in the discovery process.[10] This required increasing quantities of purified taxol, up to 600g, and in 1977 a further request for 7,000 lbs of bark was made.
In 1978, two NCI researchers published a report showing taxol was mildly effective in leukaemic mice.[11] In November 1978, taxol was shown to be effective in xenograft studies.[12] Meanwhile, taxol began to be well known in the cell biology, as well as the cancer community, with a publication in early 1979 by Susan B. Horwitz, a molecular pharmacologist at Albert Einstein College of Medicine, showing taxol had a previously unknown mechanism of action involving the stabilization of microtubules. Together with formulation problems, this increased interest from researchers meant that by 1980, the NCI envisaged needing to collect 20,000 lbs of bark.[13] Animal toxicology studies were complete by June 1982, and in November NCI applied for the IND necessary to begin clinical trials in humans.[13]
Early clinical trials, supply and the transfer to BMS
editPhase I clinical trials began in April 1984, and the decision to start Phase II trials was made a year later.[14] These larger trials needed more bark and collection of a further 12,000 pounds was commissioned, which enabled some phase II trials to begin by the end of 1986. But by then it was recognized that the demand for taxol might be substantial and that more than 60,000 pounds of bark might be needed as a minimum. This unprecedentedly large amount brought ecological concerns about the impact on yew populations into focus for the first time, as local politicians and foresters expressed unease at the program.[15]
The first public report from a phase II trial in May 1988 showed an effect in melanoma patients and a remarkable response rate of 30% in patients with refractory ovarian cancer.[16] At this point, Gordon Cragg of the NCI's Natural Product Branch calculated the synthesis of enough taxol to treat all the ovarian cancer and melanoma cases in the US would require the destruction of 360,000 trees annually. For the first time, serious consideration was given to the problem of supply.[15]
Because of the practical and, in particular, the financial scale of the program needed, the NCI decided to seek association with a pharmaceutical company, and in August 1989, it published a Cooperative Research and Development Agreement (CRADA) offering its current stock and supply from current bark stocks, and proprietary access to the data so far collected, to a company willing to commit to providing the funds to collect further raw material, isolate taxol, and fund a large proportion of clinical trials. In the words of Goodman and Welsh, authors of a substantial scholarly book on taxol,
[The NCI] was thinking, not of collaboration, ... but of a hand-over of taxol (and its problems) [17]
Although widely advertised, only four companies responded to the CRADA, including Bristol-Myers Squibb (BMS), which was selected as the partner in December 1989. The choice of BMS later became controversial and was the subject of Congressional hearings in 1991 and 1992. While it seems clear the NCI had little choice but to seek a commercial partner, there was also controversy about the terms of the deal, eventually leading to a report by the General Accounting Office in 2003, which concluded the NIH had failed to ensure value for money.[18] In related CRADAs with the USDA and Department of the Interior, Bristol-Myers Squibb was given exclusive first refusal on all Federal supplies of Taxus brevifolia. This exclusive contract lead to some criticism for giving BMS a "cancer monopoly".[19] Eighteen months after the CRADA, BMS filed a new drug application (NDA), which was given FDA approval at the very end of 1992. [17] Although there was no patent on the compound, the provisions of the Waxman-Hatch Act gave Bristol-Myers Squibb five years exclusive marketing rights.
In 1990, BMS applied to trademark the name taxol as TAXOL. This was controversially approved in 1992. At the same time, paclitaxel replaced taxol as the generic name of the compound. Critics, including the journal Nature, argued the name taxol had been used for more than two decades and in more than 600 scientific articles and suggested the trademark should not have been awarded and the BMS should renounce its rights to it.[20] BMS argued changing the name would cause confusion among oncologists and possibly endanger the health of patients. BMS has continued to defend its rights to the name in the courts.[21] BMS has also been criticized for misrepresentation by Goodman and Walsh, who quote from a company report saying
It was not until 1971 that ... testing ... enabled the isolation of paclitaxel, initially described as 'compound 17'[22]
This quote is, strictly speaking, accurate: the objection seems to be that this misleadingly neglects to explain that it was the scientist doing the isolation who named the compound taxol and it was not referred to in any other way for more than twenty years.
Annual sales peaked in 2000, reaching US$1.6 billion; paclitaxel is now available in generic form.
Production
editFrom 1967 to 1993, almost all paclitaxel produced was derived from bark from the Pacific yew, the harvesting of which kills the tree in the process. The processes used were descendants of the original isolation method of Wall and Wani; by 1987, the NCI had contracted Hauser Chemical Research of Boulder, Colorado, to handle bark on the scale needed for Phase II and III trials. While there was considerable uncertainty about how large the wild population of Taxus brevifola was and what the eventual demand for taxol would be, it had been clear for many years that an alternative, sustainable source of supply would be needed. Initial attempts used needles from the tree, or material from other related Taxus species, including cultivated ones, but these attempts were bedevilled by the relatively low and often highly variable yields obtained. It was not until the early 1990s, at a time of increased sensitivity to the ecology of the forests of the Pacific Northwest, that taxol was successfully extracted on a clinically useful scale from these sources.[23]
From the late 1970s, chemists in the US and France had been interested in taxol. A number of US groups, including one led by Robert A. Holton, attempted a total synthesis of the molecule, starting from petrochemical-derived starting materials. This work was primarily motivated as a way of generating chemical knowledge, rather than with any expectation of developing a practical production technique. By contrast, the French group of Pierre Potier at the CNRS quickly recognized the problem of yield. His laboratory was on a campus populated by the related yew Taxus baccata, so needles were available locally in large quantity. By 1981, he had shown that it was feasible to isolate relatively large quantities of the compound 10-deacetylbaccatin, a plausible first step for a semisynthetic production route to taxol. By 1988 he copublished such a semisynthetic route from needles of T. baccata.[24] The view of the NCI, however, was even this route was not practical.[25]
By 1988, and particularly with Potier's publication, it was clear to Holton as well a practical semisynthetic production route would be important. By late 1989, Holton's group had developed a semisynthetic route to paclitaxel with twice the yield of the Potier process. Florida State University, where Holton worked, signed a deal with Bristol-Myers Squibb to license this and future patents. In 1992, Holton patented an improved process with an 80% yield. BMS took the process in-house and started to manufacture paclitaxel in Ireland from 10-deacetylbaccatin isolated from the needles of the European yew.[25] In early 1993, BMS was able to announce that it would cease reliance on Pacific yew bark by the end of 1995, effectively terminating the ecological controversy over its use. This announcement also made good their commitment to develop an alternative supply route, made to the NCI in their CRADA application of 1989.
Currently, all paclitaxel production for BMS uses plant cell fermentation (PCF) technology developed by the biotechnology company Phyton Biotech, Inc and carried out at their plant in Germany.[26] This starts from a specific Taxus cell line propagated in aqueous medium in large fermentation tanks. Paclitaxel is then extracted directly, purified by chromatography and isolated by crystallization. Compared to the semisynthesis, PCF eliminates the need for many hazardous chemicals and saves a considerable amount of energy.[27]
In 1993, taxol was coincidentally discovered to be produced in a newly described fungus living in the yew tree.[28] It has since been found in a number of other endophytic fungi, including Nodulisporium sylviforme,[29] opening the possibility of taxol production by culturing one of these fungal species.
The initial motivation for synthetic approaches to paclitaxel included the opportunity to create closely related compounds. Indeed, this approach led to the development of docetaxel.
Mechanism of action
editPaclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division. Unlike other tubulin targeted drugs such as colchicine that inhibit microtubule assembly, however, paclitaxel stabilizes the microtubule polymer and protects it from disassembly. The inability of the chromosomes to achieve a metaphase spindle configuration leads to a mitotic block in which there is prolonged activation of the mitotic checkpoint with the subsequent triggering of apoptosis or slippage back into the G1-phase of the cell cycle without cell division,.[30][31] The ability of paclitaxel to inhibit spindle function is generally attributed to its suppression of microtubule dynamics;[32] but recent studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher antimitotic concentrations, paclitaxel appears to act by suppressing microtubule detachment from centrosomes, a process normally activated during mitosis.[33] The binding site for paclitaxel has been shown to reside on the beta-tubulin subunit.[34]
Clinical use
editPaclitaxel is approved in the UK for ovarian, breast and lung cancers and Kaposi's sarcoma.[35] It is recommended in NICE guidance of June 2001 that it should be used for nonsmall cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should not be used in the adjuvant treatment of early node-positive breast cancer.[36]
The cost to the NHS per patient in early breast cancer, assuming four cycles of treatment, is about $6000.[37]
Similar compounds
editThe closely related taxane docetaxel has a similar set of clinical uses to paclitaxel. It is marketed under the name of Taxotere.
Much of the clinical toxicity of paclitaxel is associated with the solvent Cremophor EL in which it is dissolved for delivery.[citation needed] Abraxis BioScience developed Abraxane, in which paclitaxel is bonded to albumin as an alternative delivery agent as an alternative to the often toxic solvent delivery method. This was approved by the Food and Drug Administration in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy.[38]
Restenosis
editPaclitaxel is used as an antiproliferative agent for the prevention of restenosis (recurrent narrowing) of coronary stents; locally delivered to the wall of the coronary artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents.[39] Paclitaxel drug eluting coated stents are sold under the trade name Taxus by Boston Scientific in the United States.
Side effects
editCommon side effects include nausea and vomiting, loss of appetite, change in taste, thinned or brittle hair, pain in the joints of the arms or legs lasting two to three days, changes in the color of the nails, and tingling in the hands or toes. More serious side effects such as unusual bruising or bleeding, pain/redness/swelling at the injection site, change in normal bowel habits for more than two days, fever, chills, cough, sore throat, difficulty swallowing, dizziness, shortness of breath, severe exhaustion, skin rash, facial flushing, female infertility by ovarian damage[40] and chest pain can also occur. A number of these side effects are associated with the excipient used, Cremophor EL, a polyoxyethylated castor oil. Allergies to drugs such as cyclosporine, teniposide and drugs containing polyoxyethylated castor oil may indicate increased risk of adverse reactions to paclitaxel.[41] Dexamethasone is given prior to beginning paclitaxel treatment to mitigate some of the side effects. Leuprolide, a GnRH analog may prevent ovarian damage, according to mice studies.[40]
Derivatives of paclitaxel
editIn recent years, extensive research has been done to find a way to mitigate the side effects of paclitaxel, by altering its administration. DHA-paclitaxel, PG-paclitaxel, and tumor-activated Taxol prodrugs are undergoing continued testing, and are actually on the way to being introduced into widespread clinical use.
Protarga has linked paclitaxel to docosahexaenoic acid (DHA), a fatty acid easily taken up by tumor cells; the DHA-paclitaxel “appears not to be cytotoxic until the bond with DHA is cleaved within the cell.”[42] The advantage of DHA-paclitaxel over paclitaxel is DHA-paclitaxel’s ability to carry much higher concentrations of paclitaxel to the cells, which are maintained for longer periods in the tumor cells, thus increasing their action. With increased activity, DHA-paclitaxel, also known as Taxoprexin, may have a more successful response in cancer patients than paclitaxel, and it may be able to treat more types of cancer than paclitaxel has been able to treat.
Cell Therapeutics has formulated PG-paclitaxel, which is paclitaxel bonded to a polyglutamate polymer; tumor cells are significantly more porous to polyglutamate polymers than normal cells, due to the leaky endothelial membranes of tumor cells. PG-paclitaxel has been introduced into clinical use, and has proven to initiate very mild side effects and to effectively treat many patients who were not responsive to the action of Taxol. The PG-paclitaxel may be a very promising anticancer drug, as it is much more selective than paclitaxel for which cells it targets.[42]
ImmunoGen has been introducing tumor-activated prodrug (TAP) technology in recent years, and is now working to apply this technology to paclitaxel. Tumor-activated Taxol prodrugs are designed for accurate targeting, by the action of a monoclonal antibody which is very specific to certain cells. Tumor-activated Taxol prodrugs research is progressing, and in mice, the “taxane-based TAP completely eradicated human tumour xenografts at non-toxic doses.”[42]
ANG1005 is made up of one molecule of a peptide called angiopep-2 joined with three molecules of paclitaxel. It is in phase I clinical trials for some types of cancer.
Research use
editAside from its direct clinical use, paclitaxel is used extensively in biological and biomedical research as a microtubule stabilizer. In vitro assays involving microtubules, such as motility assays, generally rely on paclitaxel to maintain microtubule integrity in the absence of the various nucleating factors and other stabilizing elements found in the cell. For example, it is used for in vitro tests of drugs that aim to alter the behavior of microtubule motor proteins, or for studies of mutant motor proteins. Paclitaxel is sometimes used for in vivo studies as well; it can be fed to test organisms, such as fruit flies, or injected into individual cells, to inhibit microtubule disassembly or to increase the number of microtubules in the cell.
Biosynthesis and Biocatalysis
editThis section is empty. You can help by adding to it. (July 2010) |
Additional images
edit-
Model of the paclitaxel molecule
-
Rotated paclitaxel molecule model (Animated GIF, 1.2Mb size)
References
edit- ^ Sandra Peltier, S.; Oger, J.-M., Lagarce, F.; Couet, W.; Benoît, J.-P. (2006). "Enhanced Oral Paclitaxel Bioavailability After Administration of Paclitaxel-Loaded Lipid Nanocapsules". Pharmaceutical Research. 23 (6): 1243–1250. doi:10.1007/s11095-006-0022-2. PMID 16715372. S2CID 231917.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Goodman, Jordan (2001). The Story of Taxol: Nature and Politics in the Pursuit of an Anti-Cancer Drug. Cambridge University Press. ISBN 052156123X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help), p17 - ^ Goodman and Walsh, p22
- ^ Goodman and Walsh p25, p28
- ^ a b Goodman and Walsh, p51
- ^ Wall ME, Wani MC (1995). "Camptothecin and taxol: discovery to clinic--thirteenth Bruce F. Cain Memorial Award Lecture". Cancer Res. 55 (4): 753–60. PMID 7850785.
- ^ Goodman and Walsh p51
- ^ Wani M, Taylor H, Wall M, Coggon P, McPhail A (1971). "Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia". J Am Chem Soc. 93 (9): 2325–2327. doi:10.1021/ja00738a045. PMID 5553076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goodman and Walsh p81
- ^ Goodman and Walsh p79, p81
- ^
Fuchs, David A and Johnson, Randall K (1978). "Cytologic evidence that taxol, an antineoplastic agent from Taxus brevifolia, acts as a mitotic spindle poison". Cancer Treatment Reports. 62 (8): 1219–1222. PMID 688258.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goodman and Walsh 95
- ^ a b Goodman and Walsh p97
- ^ Goodman and Walsh 115
- ^ a b Goodman and Walsh 120
- ^
Rowinsky, EK; et al. (1988). "Phase II study of taxol in advanced epithelial malignancies". Proceedings of the Association of Clinical Oncology. 7: 136.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Goodman and Walsh p120
- ^ "Technology Transfer: NIH-Private Sector Partnership in the Development of Taxol" (PDF).
- ^ Nader, Ralph; Love, James. "Looting the medicine chest: how Bristol-Myers Squibb made off with the public's cancer research." The Progressive. February, 1993. Retrieved on March 9, 2007.
- ^ "Names for hi-jacking". Nature. 373 (6513): 370. 1995. doi:10.1038/373370a0. PMID 7830775. S2CID 31510966.
- ^ Goodman and Walsh p170
- ^ Bristol-Myers Squibb, The development of TAXOL (paclitaxel), March 1997, as cited in Goodman and Walsh p2
- ^ Goodman and Walsh pp172-175
- ^ Goodman and Walsh pp100-101
- ^ a b Stephenson, Frank (Fall). "A tale of taxol". Florida State University Research in Review. 12 (3).
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help) - ^ "Phyton news release".
- ^ "2004 Greener Synthetic Pathways Award: Bristol-Myers Squibb Company: Development of a Green Synthesis for TAXOL Manufacture via Plant Cell Fermentation and Extraction".
- ^ Stierle, A., Gary Strobel, et al. (1993). "Taxol and Taxane Production by Taxomyces-Andreanae, an Endophytic Fungus of Pacific Yew." Science 260(5105): 214-216.
- ^ Zhao, K.; et al. "Study on the Preparation and Regeneration of Protoplast from Taxol-producing Fungus Nodulisporium sylviforme'." Nature and Science. 2004. 2 (2). pp. 52–59.
- ^ Bharadwaj, R., and Yu, H. (2004). The spindle checkpoint, aneuploidy, and cancer. Oncogene 23, 2016–2027.
- ^ Brito, D. A., Yang, Z., and Rieder, C. L. (2008). Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J. Cell Biol. 182, 623-629,
- ^ Jordan MA, Leslie W, Microtubules as a target for anticancer drugs Apr 4, 2004”, "[1]",
- ^ Ganguly A, Yang H, Cabral F, Paclitaxel-dependent cell lines reveal a novel drug activity.Mol Cancer Ther. 2010 Nov;9(11):2914-23. Epub 2010 Oct 26., "[2]",
- ^ Lowe, J; Li, H; Downing, KH; Nogales, E (2001). "Refined structure of αβ-tubulin at 3.5 Å resolution". Journal of Molecular Biology 313 (5): 1045–1057. doi:10.1006/jmbi.2001.5077. PMID 11700061,
- ^ Saville M, Lietzau J, Pluda J, Feuerstein I, Odom J, Wilson W, Humphrey R, Feigal E, Steinberg S, Broder S (1995). "Treatment of HIV-associated Kaposi's sarcoma with paclitaxel". Lancet. 346 (8966): 26–8. doi:10.1016/S0140-6736(95)92654-2. PMID 7603142. S2CID 44624033.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "British National Formulary".
- ^ "NICE Guidance TA108".
- ^ "Abraxane Drug Information." Food and Drug Administration. January 7, 2005. Retrieved on March 9, 2007.
- ^ Heldman A, Cheng L, Jenkins G, Heller P, Kim D, Ware M, Nater C, Hruban R, Rezai B, Abella B, Bunge K, Kinsella J, Sollott S, Lakatta E, Brinker J, Hunter W, Froehlich J (2001). "Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis". Circulation. 103 (18): 2289–95. doi:10.1161/01.cir.103.18.2289. PMID 11342479. S2CID 2143158.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ozcelik B, Turkyilmaz C, Ozgun MT; et al. (2010). "Prevention of paclitaxel and cisplatin induced ovarian damage in rats by a gonadotropin-releasing hormone agonist". Fertil. Steril. 93 (5): 1609–14. doi:10.1016/j.fertnstert.2009.02.054. PMID 19339002.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Medline Plus Entry for Paclitaxel Injection." MEDLINE. Last Reviewed 09/01/2008. Accessed 10-2-21.
- ^ a b c Whelan, Jo. Drug Discovery Today, Volume 7, Issue 2, 15 January 2002, Pages 90–92.
External links
edit- NCI Drug Information Summary for Patients.
- NCI Drug Dictionary Definition
- Molecule of the Month: TAXOL by Neil Edwards, University of Bristol.
- A Tale of Taxol from Florida State University.
- Anzatax / Paclitaxel Virtual Cancer Centre
- Berenson, Alex (October 1, 2006). "Hope, at $4,200 a Dose". The New York Times. Retrieved 2007-03-31.
- U.S. National Library of Medicine: Drug Information Portal - Paclitaxel