Uranyl fluoride is the inorganic compound with the formula UO2F2. It is most notable as a contaminant in the production of uranium tetrafluoride.[1]
| |
| Names | |
|---|---|
| IUPAC name
Uranium fluoride oxide
| |
| Other names
Uranium oxyfluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.529 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| UO2F2 | |
| Molar mass | 308.02 g/mol |
| Melting point | Decomposes @ 300 °C |
| Boiling point | Sublimes |
| Solubility in other solvents | VS |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H330, H373, H411 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
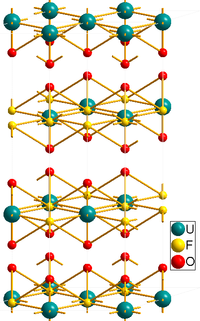
As shown by X-ray crystallography, the uranyl (UO22+) centers are complemented by six fluoride ligands.[2]
This salt is very soluble in water as well as hygroscopic. It is formed in the hydrolysis of uranium hexafluoride (UF6):
- UF6 + 2 H2O → UO2F2 + 4 HF
It can also be formed in the hydrofluorination of uranium trioxide (UO3):
- UO3 + 2 HF → UO2F2 + H2O[3]
References
edit- ^ Peehs, Martin; Walter, Thomas; Walter, Sabine; Zemek, Martin (2007). "Uranium, Uranium Alloys, and Uranium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a27_281.pub2. ISBN 978-3-527-30385-4.
- ^ Zachariasen, W. H. (1948). "Crystal chemical studies of the 5f-series of elements. III. A study of the disorder in the crystal structure of anhydrous uranyl fluoride". Acta Crystallographica. 1 (6): 277–281. Bibcode:1948AcCry...1..277Z. doi:10.1107/S0365110X48000764.
- ^ Jang, Harry; Louis-Jean, James; Poineau, Frederic (2023-06-20). "Synthesis and Morphological Control of UO2F2 Particulates". ACS Omega. 8 (24): 21996–22002. doi:10.1021/acsomega.3c01999. ISSN 2470-1343. PMC 10286299. PMID 37360455.