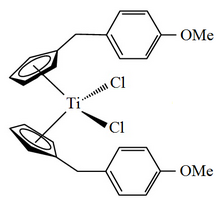
Titanocene Y also known as bis[(p-methoxybenzyl)cyclopentadienyl]titanium(IV) dichloride or dichloridobis(η5-(p-methoxybenzyl)cyclopentadienyl)titanium is an organotitanium compound that has been investigated for use as an anticancer drug.[1]
Discovery
editTitanocene dichloride is known to be a potential anticancer drug[2] since the late 1970s. After initial clinical trials against breast and renal-cell cancer were performed with this compound,[3][4] the search for improved derivatives started.[5] Particularly, lipophilic titanocene dichloride derivatives derived from fulvenes[6] were synthesised in structural diversity and this led to the development of bis[(p-methoxybenzyl)cyclopentadienyl]titanium(IV) dichloride,[1] which became better known in the literature under its trivial name of Titanocene Y.
Mechanism of action
editTitanocene Y is a cytotoxic apoptosis-inducing[7] and anti-angiogenic[8] drug candidate targeting renal-cell cancer and other solid tumors.[9][10] The compound is transported via serum albumin selectively into cancer cells[11][12] and targets their DNA by coordinating strongly to phosphate groups.[13][14] Additionally, Titanocene Y is able to induce apoptosis via the FAS receptor pathway.[15] Very encouraging is the fact that Titanocene Y is breaking platinum-resistance in human colon and human lung cancer cells,[16] which might make it attractive as a cytotoxic component of future 2nd or 3rd line cancer treatments.
Animal testing
editTitanocene Y was tested extensively in vivo; it showed promising results against xenografted human epidermoid carcinoma[17] and prostate cancer,[18] while best results are reached against breast[19] and renal-cell cancer.[20] Titanocene Y can be given in the mouse in high dosages and it shows generally mild toxicity in the form of diarrhea. Titanocene Y is not patent protected and would therefore benefit from non-commercial sponsoring to develop it into a cytotoxic drug candidate for the treatment of advanced renal-cell cancer – an area in need of better therapies.
References
edit- ^ a b Sweeney NJ, Mendoza O, Müller-Bunz H, Pampillón C, Rehmann FJ, Strohfeldt K, Tacke M (2005). "Novel benzyl substituted titanocene anti-cancer drugs". Journal of Organometallic Chemistry. 690 (21–22): 4537–4544. doi:10.1016/j.jorganchem.2005.06.039.
- ^ Köpf H, Köpf-Maier P (1979). "Titanocene dichloride--the first metallocene with cancerostatic activity". Angew. Chem. Int. Ed. Engl. 18 (6): 477–478. doi:10.1002/anie.197904771. PMID 111586.
- ^ Kröger N, Kleeberg UR, Mross K, Edler L, Hossfeld DK (2000). "Phase II Clinical Trial of Titanocene Dichloride in Patients with Metastatic Breast Cancer". Onkologie. 23 (1): 60–62. doi:10.1159/000027075. S2CID 72817279.
- ^ Lümmen G, Sperling H, Luboldt H, Otto T, Rübben H (1998). "Phase II trial of titanocene dichloride in advanced renal-cell carcinoma". Cancer Chemother. Pharmacol. 42 (5): 415–417. doi:10.1007/s002800050838. PMID 9771957. S2CID 2724249.
- ^ Allen OR, Croll L, Gott AL, Knox RJ, McGowan PC (2004). "Functionalized Cyclopentadienyl Titanium Organometallic Compounds as New Antitumor Drugs". Organometallics. 23 (2): 288–292. doi:10.1021/om030403i.
- ^ Strohfeldt K, Tacke M (2008). "Bioorganometallic fulvene-derived titanocene anti-cancer drugs". Chem Soc Rev. 37 (6): 1174–1187. doi:10.1039/b707310k. PMID 18497930.
- ^ O'Connor K, Gill C, Tacke M, Rehmann FJ, Strohfeldt K, Sweeney N, Fitzpatrick JM, Watson RW (2006). "Novel titanocene anti-cancer drugs and their effect on apoptosis and the apoptotic pathway in prostate cancer cells". Apoptosis. 11 (7): 1205–1214. doi:10.1007/s10495-006-6796-1. PMID 16699961. S2CID 31009957.
- ^ Weber H, Claffey J, Hogan M, Pampillón C, Tacke M (2008). "Analyses of Titanocenes in the spheroid-based cellular angiogenesis assay". Toxicol in Vitro. 22 (2): 531–534. doi:10.1016/j.tiv.2007.09.014. PMID 17981007.
- ^ Kelter G, Sweeney NJ, Strohfeldt K, Fiebig HH, Tacke M (2005). "In-vitro anti-tumor activity studies of bridged and unbridged benzyl-substituted titanocenes". Anticancer Drugs. 16 (10): 1091–1098. doi:10.1097/00001813-200511000-00008. PMID 16222151. S2CID 44409481.
- ^ Oberschmidt O, Hanauske AR, Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M (2007). "Antiproliferative activity of Titanocene Y against tumor colony-forming units". Anticancer Drugs. 18 (3): 317–321. doi:10.1097/CAD.0b013e3280115f86. PMID 17264765. S2CID 22670179.
- ^ Vessières A, Plamont MA, Cabestaing C, Claffey J, Dieckmann S, Hogan M, Müller-Bunz H, Strohfeldt K, Tacke M (2009). "Proliferative and anti-proliferative effects of titanium- and iron-based metallocene anti-cancer drugs". Journal of Organometallic Chemistry. 694 (6): 874–879. doi:10.1016/j.jorganchem.2008.11.071.
- ^ Lally G, Deally A, Hackenberg F, Quinn SJ, Tacke M (2013). "Titanocene Y – Transport and Targeting of an Anticancer Drug Candidate". Letters in Drug Design & Discovery. 10 (8): 675–682. doi:10.2174/15701808113100890027.
- ^ Tacke M (2008). "The Interaction of Titanocene Y with Double-Stranded DNA: A Computational Study". Letters in Drug Design & Discovery. 5 (5): 332–335. doi:10.2174/157018008784912036.
- ^ Erxleben A, Claffey J, Tacke M (2010). "Binding and hydrolysis studies of antitumoural titanocene dichloride and Titanocene Y with phosphate diesters". J. Inorg. Biochem. 104 (4): 390–396. doi:10.1016/j.jinorgbio.2009.11.010. PMID 20036426.
- ^ Kater L, Claffey J, Hogan M, Jesse P, Kater B, Strauss S, Tacke M, Prokop A (2012). "The role of the intrinsic FAS pathway in Titanocene Y apoptosis: The mechanism of overcoming multiple drug resistance in malignant leukemia cells". Toxicol in Vitro. 26 (1): 119–124. doi:10.1016/j.tiv.2011.09.010. PMID 21986259.
- ^ Hilger A, Alex D, Deally A, Gleeson B, Tacke M, Ralf (2011). "Titanocene Y and Vanadocene Y: Platinum Resistance-Breaking Cytotoxic and DNA-Targeting Anticancer Drug Candidates". Letters in Drug Design & Discovery. 8 (10): 904–910. doi:10.2174/157018011797655241.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bannon JH, Fichtner I, O'Neill A, Pampillón C, Sweeney NJ, Strohfeldt K, Watson RW, Tacke M, Mc Gee MM (2007). "Substituted titanocenes induce caspase-dependent apoptosis in human epidermoid carcinoma cells in vitro and exhibit antitumour activity in vivo". Br. J. Cancer. 97 (9): 1234–1241. doi:10.1038/sj.bjc.6604021. PMC 2360460. PMID 17923871.
- ^ Dowling CM, Claffey J, Cuffe S, Fichtner I, Pampillón C, Sweeney NJ, Strohfeldt K, Watson RW, Tacke M (2008). "Antitumor activity of Titanocene Y in xenografted PC3 tumors in mice". Letters in Drug Design & Discovery. 5 (2): 141–144. doi:10.2174/157018008783928463.
- ^ Beckhove P, Oberschmidt O, Hanauske AR, Pampillón C, Schirrmacher V, Sweeney NJ, Strohfeldt K, Tacke M (2007). "Antitumor activity of Titanocene Y against freshly explanted human breast tumor cells and in xenografted MCF-7 tumors in mice". Anticancer Drugs. 18 (3): 311–315. doi:10.1097/CAD.0b013e328010a6f7. PMID 17264764. S2CID 42898975.
- ^ Fichtner I, Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M (2006). "Antitumor activity of Titanocene Y in xenografted CAKI-1 tumors in mice". Anticancer Drugs. 17 (3): 333–336. doi:10.1097/00001813-200603000-00012. PMID 16520662. S2CID 45195878.
External links
edit- Titanocene Y at the U.S. National Library of Medicine Medical Subject Headings (MeSH)