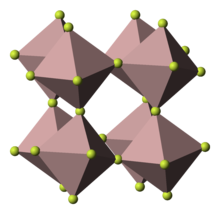
Titanium(III) fluoride is the inorganic compound with the formula TiF3. A violet, paramagnetic solid, it is one of two titanium fluorides, the other being titanium tetrafluoride.[1] It adopts a defect perovskite-like structure such that each Ti center has octahedral coordination geometry, and each fluoride ligand is doubly bridging.[2]
| |

| |
| Names | |
|---|---|
| IUPAC name
Titanium(III) fluoride
| |
| Other names
Titanium trifluoride
Titanous fluoride Trifluorotitanium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.379 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TiF3 | |
| Molar mass | 104.862 g/mol |
| Appearance | violet to purple-red powder |
| Density | 2.98 g/cm3 |
| Melting point | 1,200 °C (2,190 °F; 1,470 K) |
| Boiling point | 1,400 °C (2,550 °F; 1,670 K) |
| soluble | |
| +1300·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P280, P305+P351+P338, P310 | |
| Related compounds | |
Other anions
|
Titanium(III) bromide Titanium(III) chloride Titanium(III) iodide |
Related compounds
|
Titanium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium(III) fluoride can be prepared by dissolution of titanium metal in hydrogen fluoride. In air, it slowly oxidizes to titanium(IV).[1]
References
edit- ^ a b Meshri, Dayal T. (2000). "Fluorine Compounds, Inorganic, Titanium". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2009200113051908.a01. ISBN 978-0-471-48494-3.
- ^ H. Sowa; H. Ahsbahs (1998). "Pressure-Induced Octahedron Strain in VF3-Type Compounds". Acta Crystallogr. B54 (5): 578–584. Bibcode:1998AcCrB..54..578S. doi:10.1107/S0108768198001207.