Tin(IV) sulfide is a compound with the formula Sn S
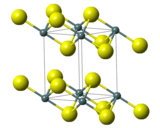
2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers.[5] It occurs naturally as the rare mineral berndtite.[6] It is useful as semiconductor material with band gap 2.2 eV.[7]
| |
| Names | |
|---|---|
| IUPAC name
Tin(IV) sulfide
| |
| Other names
Tin disulfide, Stannic sulfide, Mosaic gold
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ECHA InfoCard | 100.013.867 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| S2Sn | |
| Molar mass | 182.83 g·mol−1 |
| Appearance | Gold-yellow powder |
| Odor | Odorless |
| Density | 4.5 g/cm3[1] |
| Melting point | 600 °C (1,112 °F; 873 K) decomposes[1] |
| Insoluble | |
| Solubility | Soluble in aq. alkalis, decompose in aqua regia[1] Insoluble in alkyl acetates, acetone[2] |
| Structure | |
| Rhombohedral, hP3[3] | |
| P3m1, No. 164[3] | |
| 3 2/m[3] | |
a = 3.65 Å, c = 5.88 Å[3] α = 90°, β = 90°, γ = 120°
| |
| Octahedral (Sn4+)[3] | |
| Hazards | |
| GHS labelling: | |
 [4] [4]
| |
| Warning | |
| H302, H312, H315, H319, H332, H335[4] | |
| P261, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P332+P313[4] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reactions
editThe compound precipitates as a brown solid upon the addition of H
2S to solutions of tin(IV) species. This reaction is reversed at low pH. Crystalline SnS
2 has a bronze color and is used in decorative coating[8] where it is known as mosaic gold.
The material also reacts with sulfide salts to give a series of thiostannates with the formula [SnS
2]
m[S]2n−
n. A simplified equation for this depolymerization reaction is
- SnS
2 + S2−
→ 1/x[SnS2−
3]
x.
Applications
editTin (IV) sulfide has various uses in electrochemistry. It can be used in anodes of lithium-ion batteries, where an intercalation process occurs to form Li2S.[9] It can also be used in a similar way in electrodes of supercapacitors, which can be used as alternative source of energy storage.[10]
SnS2 has also been identified as a potential component of thermoelectric devices, which convert thermal energy to electrical energy. In one example, this property was made possible by forming a composite of SnS2 with multiwalled carbon nanotubes.[11]
SnS2 can also be used in wastewater treatment. Forming a membrane with SnS2 and carbon nanofibers can potentially allow for the reduction of certain impurities in water, an example of which is hexavalent chromium.[12]
In general, SnS2 is useful as a semiconductor and can be purchased in powder form for experimental purposes.[13]
See also
editReferences
edit- ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 1080.
- ^ a b c d e Voort, G.F. Vander, ed. (2004). "Crystal Structure*" (PDF). ASM Handbook. 9 (Metallography and Microstructures): 29–43. doi:10.1361/asmhba0003722 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link)[permanent dead link] - ^ a b c d "SDS of Stannic sulfide" (PDF). pfaltzandbauer.com. Connecticut, USA: Pfaltz & Bauer, Inc. Archived from the original (PDF) on 2014-07-14. Retrieved 2014-07-13.
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Vaughan, D. J.; Craig, J. R. "Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge: 1978. ISBN 0-521-21489-0.
- ^ L.A.Burton et al., J. Mater. Chem. A, 2016, 4, 1312-1318 DOI: 10.1039/C5TA08214E.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Cupid, D. M.; Rezqita, A.; Glibo, A.; Artner, M.; Bauer, V.; Hamid, R.; Jahn, M.; Flandorfer, H. (2021). "Understanding and Modelling the Thermodynamics and Electrochemistry of Lithiation of Tin (IV) Sulfide as an Anode Active Material for Lithium Ion Batteries". Electrochim. Acta. 375. doi:10.1016/j.electacta.2021.137936.
- ^ Setayeshmehr, M.; Haghighi, M.; Mirabbaszadeh, K. (2021). "A Review of Tin Disulfide (SnS2) Composite Electrode Materials for Supercapacitors". Energy Storage. 4.
- ^ Park, D.; Kim, M.; Kim, J. (2022). "Strongly Coupled Tin(IV) Sulfide—MultiWalled Carbon Nanotube Hybrid Composites and Their Enhanced Thermoelectric Properties". Inorg. Chem. 61 (8): 3723–3729. doi:10.1021/acs.inorgchem.1c03953. PMID 35179362. S2CID 246944069.
- ^ Zhong, Y.; Qiu, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. (2016). "Flexible Electrospun Carbon Nanofiber/Tin(IV) Sulfide Core/Sheath Membranes for Photocatalytically Treating Chromium(VI)-Containing Wastewater". ACS Appl. Mater. Interfaces. 8 (42): 28671–28677. doi:10.1021/acsami.6b10241. PMID 27723961.
- ^ "Tin (IV) Sulfide (SnS2) Powder/Chunk/Lumps (CAS No.1315-01-1) | Stanford Advanced Materials". www.samaterials.com. Retrieved 2023-11-21.
