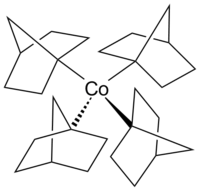
Tetrakis(1-norbornyl)cobalt(IV) is an air-sensitive organometallic compound of cobalt. It was first synthesized by Barton K. Bower and Howard G. Tennent in 1972[1] and is one of few compounds in which cobalt has a formal oxidation state of +4.
| |
| Names | |
|---|---|
| Other names
(T-4)-Tetrakis(bicyclo[2.2.1]hept-1-yl)cobalt
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C28H44Co | |
| Molar mass | 439.593 g·mol−1 |
| Appearance | brown crystals |
| Melting point | 100 °C (decomposes) |
| Solubility | soluble in THF |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editTetrakis(1-norbornyl)cobalt(IV) is formed the reaction of CoCl2•THF with 1-norbornyllithium (norLi) in n-pentane under an inert atmosphere.[1] The cobalt(II) chloride-THF adduct is prepared from Soxhlet extraction of anhydrous CoCl2 with THF, and the organolithium reagent is prepared from the reaction between 1-chloro-norbornane and lithium metal.
The compound can then be purified by recrystallization.
Properties
editThe complex is a thermally stable homoleptic tetraorganylcobalt(IV) complex with exclusively σ-bonding ligands. It was the first low-spin complex with tetrahedral geometry to be isolated.[2][3][4]
Stability
editThe exceptional stability of the complex is in large part due to its inability to undergo either α- or β-hydride elimination. The α-position of the metal (corresponding to the 1-position of the norbornyl ligand) has no more hydrogen atoms, while hydride elimination from the β-position would yield an energetically unfavorable double bond on a bridgehead atom (Bredt's rule). Moreover, the bulky norbornyl ligands sterically shield the central atom, hindering ligand substitutions as well as homolysis.[1][5]
The rare d5 low-spin configuration in a tetrahedral ligand field is possible because the ligand is so strongly σ-donating that the gap between the e und t2 orbitals is raised sufficiently to overcome the spin pairing energy. The resulting configuration is e4t21, with magnetic measurements showing paramagnetism consistent with only one unpaired electron.[1][3][4]
Cobalt(III) and cobalt(V) derivatives
editThe reaction between CoCl2•THF and 1-norbornyllithium (norLi) also allows the formation of a cobalt(III) complex: if a mixture of diethyl ether and THF is used as the solvent in place of n-pentane, the resulting disproportionation reaction affords the complex tetrakis(1-norbornyl)cobaltate(III), which crystallizes out of solution with solvated lithium counterions, along with elemental cobalt.[4][6]
The compound is air-sensitive, has a green color and is paramagnetic, with two unpaired electrons, again indicating a low-spin tetrahedral configuration (d6, e4t22).[6][4]
The corresponding cobalt(V) complex is prepared by oxidizing tetrakis(1-norbornyl)cobalt(IV) with Ag[BF4] in THF and crystallizes with tetrafluoroborate as the counterion.[6][4]
- Co(nor)4 + AgBF4 → [Co(nor)4]BF4 + Ag
This complex :[Co(nor)4]+ is the first cobalt(V) complex to be isolated. Again the configuration is low-spin (d4, e4t20).[2][4][6]
See also
editReferences
edit- ^ a b c d B. K. Bower and H. G. Tennent (1972). "Transition metal bicyclo[2.2.1]hept-1-yls". J. Am. Chem. Soc. 94 (7): 2512–2514. doi:10.1021/ja00762a056.
- ^ a b Holleman, Arnold F.; Wiberg, E.; Wiberg, N. (2007). Lehrbuch der anorganischen Chemie (102nd ed.). Berlin. p. 1695. ISBN 978-3-11-017770-1. OCLC 180963521.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b E. K. Byrne, D. S. Richeson and K. H. Theopold (1986). "Tetrakis(1-norbornyl)cobalt, a low spin tetrahedral complex of a first row transition metal". J. Chem. Soc., Chem. Commun. (19): 1491–1492. doi:10.1039/C39860001491.
- ^ a b c d e f E. K. Byrne, K. H. Theopold (1989). "Synthesis, characterization, and electron-transfer reactivity of norbornyl complexes of cobalt in unusually high oxidation states". J. Am. Chem. Soc. 111 (11): 3887–3896. doi:10.1021/ja00193a021.
- ^ Riedel, Erwin; Alsfasser, R.; Janiak, C.; Klapötke, T. M.; Meyer, H.-J. (2007). Moderne Anorganische Chemie. Berlin • New York: Walter de Gruyter. p. 718. doi:10.1515/9783110206852. ISBN 978-3-11-020685-2.
- ^ a b c d E. K. Byrne, K. H. Theopold (1987). "Redox chemistry of tetrakis(1-norbornyl)cobalt. Synthesis and characterization of a cobalt(V) alkyl and self-exchange rate of a Co(III)/Co(IV) couple". J. Am. Chem. Soc. 109 (4): 1282–1283. doi:10.1021/ja00238a066.
External links
edit- Bower, Barton K. (12 December 1972). "Tetra(bicycloheptyl) transition metal compounds".