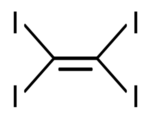
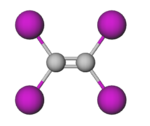
Tetraiodoethylene (TIE), or diiodoform, is the periodinated analogue of ethylene with the chemical formula C2I4. It is a decomposition product of carbon tetraiodide and diiodoacetylene.[5] It is an odourless yellow crystalline solid that is soluble in benzene and chloroform, and insoluble in water.[2] It has been used as an antiseptic and a component in pesticide and fungicide formulations.[6][7]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tetraiodoethene
| |||
| Other names
Periodoethylene, Periodoethene, Diiodoform, Ethylene tetraiodide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.434 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2I4 | |||
| Molar mass | 531.640 g·mol−1 | ||
| Appearance | yellow crystalline solid | ||
| Odor | Odourless[1] | ||
| Density | 2.98 | ||
| Melting point | 187–192 °C (369–378 °F; 460–465 K)[2] | ||
| Boiling point | Sublimates[2] | ||
| Insoluble | |||
| Solubility | Soluble in chloroform, carbon disulphide, benzene and toluene[3] | ||
| Hazards | |||
| GHS labelling:[4] | |||

| |||
| Warning | |||
| H302, H312, H315, H319, H332, H335 | |||
| P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetraiodoethylene reacts with ethylamine to give ethylamine di-tetraiodoethylene, EtNH2.(C2I4)2, and ethylaminetetraiodoethylene.[8] Tetraiodoethylene and iodine pentafluoride yield iodopentafluoroethane.[9]
Tetraiodoethylene turns brown and emits a "characteristic" odour due to decomposition when exposed to light.[10]
History
editTetraiodoethylene was discovered by Baeyer in 1885.[5] It was proposed as an antiseptic under the name Diiodoform, in 1893 by M. L. Maquenne and Taine.[10] It was an alternative to iodoform[10] which has a strong and persistent odour that caused difficulties for physicians in private practices.[11]
Synthesis
editTetraiodoethylene can be made by the iodination of calcium carbide:[1]
- CaC2 + 3I2 → C2I4 + CaI2
Diiodoacetylene is a byproduct of the reaction which can later be iodinated to TIE.[1]
The action of aqueous solution of potassium hydroxide and iodine on barium carbide in chloroform or benzene can also give TIE.[10] Another synthesis involves mixing separate solutions of diiodoacetylene and iodine in carbon disulphide. Tetraiodoethylene would be left as a residue after carbon disulphide was evaporated.[10]
See also
editReferences
edit- ^ a b c TETRA-IODO-ETHYLENE, The Pharmaceutical Era. (1897). USA, D. O. Haynes & Company.
- ^ a b c Lide, D. R. (1995). CRC Handbook of Chemistry and Physics. (Special Student Edition): A Ready-Reference Book of Chemical and Physical Data. CRC-Press. Page 522
- ^ a b Diiodoform in Notes on New Remedies. (1893). USA: Lehn & Fink.
- ^ "Tetraiodoethylene". pubchem.ncbi.nlm.nih.gov.
- ^ a b Peculiar Decomposition of Diiodoacetylene, Victor Meyel and Wilhelm Pemsel (1896)
- ^ The Code of Federal Regulations of the United States of America (1984). U.S. Government Printing Office.
- ^ Iodine and Plant Life: Annotated Bibliography, 1813-1949. With Review of the Literature. (1950). UK: Chilean Iodine Educational Bureau. page 22
- ^ Action of Tetraiodoethylene on Organic Bases, Chemical Abstracts. (1912). American Chemical Society.
- ^ Journal of the Chemical Society. (1948). page 2188
- ^ a b c d e New Remedies – Diiodoform in Merck's Market Report and Pharmaceutical Journal: An Independent Monthly Magazine Devoted to the Professional and Commercial Interests of the Druggist. (1894).
- ^ Selections – DIIODOFORM in The International Dental Journal. (1895). USA: International Dental Publication Company.