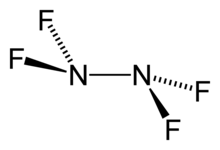
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable,[2] reactive inorganic gas. It is a fluorinated analog of hydrazine.
| |
| |
| Names | |
|---|---|
| IUPAC name
1,1,2,2-tetrafluorohydrazine
| |
| Other names
Tetrafluorohydrazine, perfluorohydrazine, UN 1955
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.091 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| N2F4 | |
| Molar mass | 104.008 g·mol−1 |
| Appearance | Colourless gas |
| Melting point | −164.5 °C (−264.1 °F; 108.6 K)[1] |
| Boiling point | −73 °C (−99 °F; 200 K)[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Explosion |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
10 mL/kg (rat, intraperitoneal)[2] |
LC50 (median concentration)
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editTetrafluorohydrazine was originally prepared from nitrogen trifluoride using a copper as a fluorine atom acceptor:[3]
- 2NF3 + Cu → N2F4 + CuF2
A number of F-atom acceptors can be used, including carbon, other metals, and nitric oxide. These reactions exploit the relatively weak N-F bond in NF3.[4]
Properties
editTetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride.[5]
- N2F4 ⇌ 2 •NF2
At room temperature N2F4 is mostly associated with only 0.7% in the form of NF2 at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF2.[6]
The energy needed to break the N−N bond in N2F4 is 20.8 kcal/mol, with an entropy change of 38.6 eu.[6] For comparison, the dissociation energy of the N−N bond is 14.6 kcal/mol (61 kJ/mol) in N2O4, 10.2 kcal/mol (43 kJ/mol) in N2O2, and 60 kcal/mol (250 kJ/mol) in N2H4. The enthalpy of formation of N2F4 (ΔfH°) is 34.421 kJ/mol.[7]
Uses
editTetrafluorohydrazine is used in organic synthesis and some rocket propellant formulations.[2] It adds across double bonds to give vicinal di(difluoroamine)s.[8] In chemical syntheses, as a precursor or a catalyst. It was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959.[9]
Safety
editTetrafluorohydrazine is a highly hazardous chemical that explodes in the presence of organic materials.[2]
It is a toxic chemical which irritates skin, eyes and lungs. It is a neurotoxin and may cause methemoglobinemia. It may be fatal if inhaled or absorbed through skin. Vapors may be irritating and corrosive. It is a strong oxidizing agent. Contact with this chemical may cause burns and severe injury. Fire produces irritating, corrosive and toxic gases. Vapors from liquefied gas are initially heavier than air and spread across the ground.[2]
Tetrafluorohydrazine explodes or ignites on contact with reducing agents at room temperature, including hydrogen, hydrocarbons, alcohols, thiols, amines, ammonia, hydrazines, dicyanogen, nitroalkanes, alkylberylliums, silanes, boranes or powdered metals. Prolonged exposure of the container of tetrafluorohydrazine to high heat may cause it to rupture violently and rocket. Tetrafluorohydrazine itself can explode at high temperatures or with shock or blast when under pressure. When heated to decomposition in air, it emits highly toxic fumes of fluorine and oxides of nitrogen.[2]
There is a fatal case in which during opening of valves to check the pressure, the cylinder exploded, killing one man and injuring another.[2]
References
edit- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c d e f g h "Tetrafluorohydrazine". pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Retrieved 26 March 2023. This article incorporates text from this source, which is in the public domain.
- ^ Ruff, John K. (1967). "Derivatives of Nitrogen Fluorides". Chemical Reviews. 67 (6): 665–680. doi:10.1021/cr60250a004.
- ^ Clark, John Drury (1972). Ignition!. New Brunswick, NJ: Rutgers UP. p. 82. ISBN 0-8135-0725-1. LCCN 72-185390.
- ^ Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans; Wagner, Joachim (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Ruprecht, Sigrid; Wagner, Joachim (eds.). F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. Vol. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. Retrieved 29 August 2015.
- ^ a b Bohn, Robert K.; Bauer, Simon Harvey (February 1967). "An electron diffraction study of the structures of NF2 and N2F4". Inorganic Chemistry. 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- ^ "Nitrogen difluoride NF2(g)". www.chem.msu.su.
- ^ Clark 1972, pp. 167–168.
- ^ Tetrafluorohydrazine at DTIC.mil archived March 12, 2007