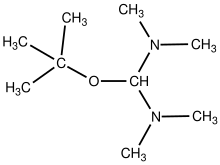
tert-Butoxybis(dimethylamino)methane is an organic compound with the formula (CH3)3COCH(N(CH3)2)2. The compound is classified as an aminal ester, i.e. the tert-butyl alcohol derivative of the aminal bis(dimethylamino)methane. It is a colorless liquid with a amine odor.
| |
| Names | |
|---|---|
| Other names
Bredereck's reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.024.895 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H22N2O | |
| Molar mass | 174.288 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 50-52°C at 12 mmHg |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Use as reagent
editAlso known as Bredereck's reagent, it is used for formylation (introduction of the CHO group).[1] Protonation releases tert-butyl alcohol, giving tetramethylformamidinium, which displaces active C-H bonds:
- (CH3)3COCH(N(CH3)2)2 + H+ → (CH3)3COH + [CH(N(CH3)2)2]+
- [CH(N(CH3)2)2]+ + CH2Z2 → Z2CHCH(N(CH3)2)2 + H+
The resulting bis(dimethylamino)methyl derivative in turn releases dimethylamine to give an enamine, which hydrolyzes.
- Z2CHCH(N(CH3)2)2 → Z2C=CHN(CH3)2 + HN(CH3)2
- Z2C=CHN(CH3)2 + H2O → Z2CHCHO + HN(CH3)2
Preparation
editTert-Butoxybis(dimethylamino)methane is obtained from tetramethylformamidinium chloride by reaction with tert-butoxide. Tetramethylformamidinium salts are obtained by the reaction of dimethylformamide (DMF) with dimethylcarbamoyl chloride[2]
References
edit- ^ Kantlehner, Willi; Bowers, Albert (2007). "T-Butoxybis(dimethylamino)methane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/9780470842898.rb350.pub2. ISBN 978-0471936237.
- ^ Arnold, Z. (1959). "The preparation of tetramethylformamidinium salts and their vinylogues". Collection of Czechoslovak Chemical Communications. 24 (3): 760–765. doi:10.1135/cccc19590760.