The noble gases are a group of chemical elements with similar properties in the periodic table. The six noble gases that occur naturally are helium, neon, argon, krypton, xenon, and the radioactive radon. Under standard conditions, the gases are all colorless, odorless, tasteless and nonflammable. The noble gases show extremely low chemical reactivity, and only a few hundred noble gas compounds have been formed.
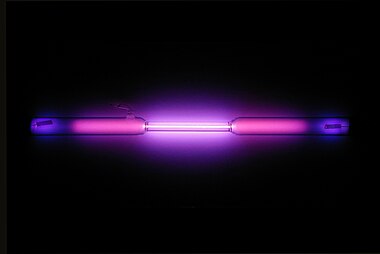
This picture shows a gas discharge tube containing argon.
See images of other noble gases: Helium · Neon · KryptonPhotograph: Alchemist-hp