Talk:Titanium dioxide
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| The contents of the Titanium dioxide (B) page were merged into Titanium dioxide on 11:46, 26 November 2009. For the contribution history and old versions of the redirected page, please see its history; for the discussion at that location, see its talk page. |
Wiki Education Foundation-supported course assignment
editThis article is or was the subject of a Wiki Education Foundation-supported course assignment. Further details are available on the course page. Peer reviewers: ChrisRoberts96.
Above undated message substituted from Template:Dashboard.wikiedu.org assignment by PrimeBOT (talk) 11:25, 17 January 2022 (UTC)
Untitled
editThe values refers to STP, but it says 0°C (273,15 K). In Europe I think that is 25°C. Either STP is wrong or please clear this out for me. :-) (What is the "standard temperature"?) // Rogper 10:41, 16 Feb 2004 (UTC)
Is titanium dioxide desirable or dangerous in products such as vitamin tablets? — Preceding unsigned comment added by 151.198.55.167 (talk • contribs) 00:09, 9 April 2004
titanium dioxide is nontoxic - it is used frequently in toothpaste and food, as well as cosmetics and skin care (which i just added)
i just rewrote the "uses of" paragraph to include more information and to clarify things. as this paragraph discusses both properties of Ti02 and uses of, should it be split?
--empup — Preceding unsigned comment added by 64.30.194.196 (talk • contribs) 20:20, 9 October 2004
Does anyone know how to fix the properties table so it doesn't disappear off the bottom of the screen? Isn't it supposed to be alongside the text, as it is with the element pages? I don't know the code well enough to fix this. Walkerma 21:21, 19 Jan 2005 (UTC)
"Recently (1992)" sounds a bit funny. Any update — Preceding unsigned comment added by 80.136.153.123 (talk • contribs) 08:01, 23 October 2005
Has there been any research done linking cancer to titanium dioxide found in food?
--redfella — Preceding unsigned comment added by 66.49.88.154 (talk • contribs) 13:53, 26 September 2006
At least there is evidence in one publication that titanium dioxide in sunscreen leads to allergic reactions and Japanese found that titanium implants led to dermatitis. I think that the mucosa of the intestine and of the stomach should be classified as "skin". — Preceding unsigned comment added by 84.147.163.53 (talk) 15:09, 18 June 2012 (UTC)
there is sound scientific proof that small particles of TiO2 trigger inflammation and probably cancer (↑ A. S. Yazdi, G. Guarda, N. Riteau, S. K. Drexler, A. Tardivel, I. Couillin, J. Tschopp: Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1? and IL-1?. In: Proceedings of the National Academy of Sciences of the United States of America. Band 107, Nummer 45, November 2010, S. 19449–19454, ISSN 1091-6490. doi:10.1073/pnas.1008155107. PMID 20974980. PMC 2984140.) — Preceding unsigned comment added by 84.147.178.51 (talk • contribs) 21:43, 16 June 2012
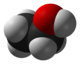
The structural graphic of Titanium Oxide does not match the formula listed, as the ratio of entities shown in the graphic is 6:9. — Preceding unsigned comment added by Sptsailing (talk • contribs) 03:04, 10 September 2014 (UTC)
- Well I also see 6 red and 9 grey balls. BUT you should only count the balls inside the box. There is one grey ball fully inside the box. The 8 balls at the corners of the box have only a small part inside. If you combine the small parts they will make a second grey ball. Now you have two grey balls. There are two red balls balls fully inside the box. The two upper red balls are only halfways inside the box and the same goes for the two lower ones. So there are 2 balls plus two halves (upper) plus two halves (lower), all together 4 red balls. The graphic shows two formula units of TiO2 ie the ratio is correct.Stenallan (talk) 15:49, 9 April 2015 (UTC)
Other TiO2 uses
editTitanium dioxide is also used extensively in the handmade soap crafts as a colorant/pigment. It was interesting to find out that it is not a naturally occurring mineral/metal.
--- Paul E. Achuff, Sr. — Preceding unsigned comment added by 68.234.74.59 (talk • contribs) 20:53, 16 November 2005
- Titanium dioxide does occur naturally (see rutile), but not in sufficient quantities or purity to make this a reasonable commercial source. Physchim62 (talk) 04:49, 17 November 2005 (UTC)
Working on Osseointegration I discovered that the mechanism by which titanium bonds with human bone in surgical implants is through the "titanium oxide'" layer of the implant. I'm not really sure if this constitutes a "use" or a property, or what. mordicai. 01:36, 17 September 2006 (UTC)
Industrial Economics
editTitanium Dioxide is listed many times as an example (with Dupont) in industrial economics, relating to entry deterrance, amongst other things. This should really be noted, however I don't know enough about the situation(s) to mention it, however there are journal articles out there for anyone with the time/inclination to add it...
Ant — Preceding unsigned comment added by 80.229.137.252 (talk • contribs) 19:43, 14 April 2006
How is this formed?
editI am doing a sciende fair project over lipsticks. In order to make the color pink, you must mix titanium oxide and various shades of D&C red no. 21-27 -ish it depends on how dark of lite you want it.
My question is How Does Titanium Oxide form, is it the gas that is released or is it a chemcial reaction with another element?
Please help me.
You would like to.
Much appreaciated.
That science nerd, Katie 7th grade. — Preceding unsigned comment added by 66.68.55.106 (talk • contribs) 04:11, 30 November 2006
- Titanium dioxide is made by reaction of titanium tetrachloride with water, as described here. I know this first hand - the chemical plant I used to work at was near a big TiO2 plant, and there was a TiO2 "snow" lying all around the plant from years of them running that reaction! TiCl4 itself is a liquid, so the reaction with water can produce a fog of TiO2 under some conditions (see Titanium_tetrachloride#Smoke-screens). I presume that the reaction produces a fairly consistent particle size, as needed by the paint industry, this would not be the case it you simply tried to take rutile and grind it up. Walkerma 15:58, 30 November 2006 (UTC)
- Titanium dioxide is found in the ground as the minerals anatase and rutile. However, it must be purified before it can be used in paints or lipsticks, because the natural form contains to much iron. Physchim62 (talk) 18:13, 30 November 2006 (UTC)
Anti-Pollutant
editI updated Jubilee Church to reflect information from ABC News that titanium dioxide can reduce smog. I'll leave the boldness to the chemists: [1] —The preceding unsigned comment was added by Freder1ck (talk • contribs) 19:51, 3 December 2006 (UTC).
Refractive Index
editBy far the most important use of titanium dioxide is as a white pigment. Reason being, titanium dioxide has a very high refractive index. Simply speaking, the higher the reflectance the greater the ability to scatter light. The author has stated the refractive index as 2.4. This is only partialy correct. The refractive index of the anatase crystal is 2.4 while that of the rutile crystal is 2.7. It should be no surprise then, the rutile form of titanium dioxide is most predominantly used in paints and plastics.
Generally speaking, this difference in refractvive index provides for rutile titaniun dioxide to be 30% more efficient than anatase in a paint or plastics. —The preceding unsigned comment was added by 68.39.4.210 (talk) 19:41, 10 December 2006 (UTC).
Elastic module
editA single value is given for the elastic modulus. This is ambiguous to me for two reasons: firstly, is the number given for rutile or anatase? Secondly, both rutile and anatase are anisotropic materials, and therefore they will have different moduli along the different axes of their crystals. Another, less important reason why this is ambiguous, is that the name "elastic modulus" isn't as specific as it could be. I know it probably means Young's modulus, but it would be nice to just say that. Ed Sanville 13:12, 17 January 2007 (UTC)
Titanium dioxide in cosmetic products
editConsidering TO2's potential as an oxidiser and producer of free radicals, it's a concern that we use this product so extensively on our skin. It will be interesting to see if there is any future correlation between the use of TO2 and development of skin cancer. Especially as it is often formulated in sunscreens as a 'safe' alternative to chemical UV absorbers. 58.7.57.55 02:50, 14 April 2007 (UTC)GBS
- That's why the cosmetic grade is doped with (IIRC) manganese to quench the excited state. 70.16.205.62 03:12, 26 July 2007 (UTC)
Used as a semiconductor
editThe ref is very old (1941)- is TiO2 really used as a semi-conductor or has it just got semi-conducting properties?--Axiosaurus (talk) 16:32, 4 May 2008 (UTC)
WikiProject Food and drink Tagging
editThis article talk page was automatically added with {{WikiProject Food and drink}} banner as it falls under Category:Food or one of its subcategories. If you find this addition an error, Kindly undo the changes and update the inappropriate categories if needed. The bot was instructed to tagg these articles upon consenus from WikiProject Food and drink. You can find the related request for tagging here . Maximum and careful attention was done to avoid any wrongly tagging any categories , but mistakes may happen... If you have concerns , please inform on the project talk page -- TinucherianBot (talk) 01:22, 4 July 2008 (UTC)
Titanium Dioxide Allergies
editI, in the last few years, have discovered an increasing reaction to Titanium Dioxide. It was due to an earlier article here I was finally able to purge it from my life. The edited version now has enlightened me even more. Though I have minor reactions to Zinc, Aluminum and Tin Oxides, my Titanium Dioxide allergy stumped my allergist and she was amazed I was able to figure it out. Thank you for continuing to build on this page to help people like me! ---Allergy Misfit —Preceding unsigned comment added by Titaniumallergies (talk • contribs) 03:41, 25 August 2008 (UTC)
It does not trigger a true allergy but an inflammatory response (see inflamasome) which leads to cell death and possibly cancer (↑ A. S. Yazdi, G. Guarda, N. Riteau, S. K. Drexler, A. Tardivel, I. Couillin, J. Tschopp: Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1? and IL-1?. In: Proceedings of the National Academy of Sciences of the United States of America. Band 107, Nummer 45, November 2010, S. 19449–19454, ISSN 1091-6490. doi:10.1073/pnas.1008155107. PMID 20974980. PMC 2984140.)The inflammasome reaction has also been proven for siliciumdioxide and asbestos. — Preceding unsigned comment added by 84.147.178.51 (talk • contribs) 21:43, 16 June 2012
Electrical properties
editWhy are all the electrical properties of titanium dioxide missing? I tried (unsuccessfully) to add them to the Chembox. Can anyone help? 70.56.100.7 (talk)
Plagiarism
editIt seems the entire toxicology section has been copied verbatim from this page: http://www.ccohs.ca/headlines/text186.html —Preceding unsigned comment added by 205.210.232.62 (talk) 18:23, 15 December 2008 (UTC)
Removed link
editI removed this line from the external links section:
- [2] "Diflucan antifungal med has titanium dioxide "inactive ingregient" in suspension - see page 1 of linked full prescribing info from Pfizer". Is it carcinogenic like the powder breathing is?
The answer is no; see the article and various sections of this talk page. --ἀνυπόδητος (talk) 19:06, 28 December 2008 (UTC)
Titania is the only material which can be used in lot of applications —Preceding unsigned comment added by 210.212.152.98 (talk) 09:50, 11 February 2010 (UTC)
It has band gap of 3.2ev,it can be used as a photocatalyst in decontamination of toxic chemicals in presence of UV light. —Preceding unsigned comment added by 210.212.152.98 (talk) 09:52, 11 February 2010 (UTC)
Solubility
editI think the solubility is wrong, 7.4 times 10 to the 5 molars?! Titanium dioxide is insoluble in water! Maybe it's negative 5? — Preceding unsigned comment added by 76.66.48.56 (talk • contribs) 00:24, 5 December 2009
- Sure. Thanks. Removed. Materialscientist (talk) 00:28, 5 December 2009 (UTC)
there's 2 kinds, hydrophobic and nonhydrophobic, hydrophobic is produce with a oil mix in it at the final stage of production and therefor will not mix with water, only oil base products —Preceding unsigned comment added by 52.129.8.48 (talk) 18:53, 26 March 2010 (UTC)
logic of Titanium Dioxide in milk
editSince inhaling Ti02 dust causes lung cancer, it seems that the common event of people having on occasion food going down the wind pipe would open lung cancer from Skim Milk as a risk. —Preceding unsigned comment added by 71.114.180.207 (talk) 03:24, 17 September 2010 (UTC)
Doesn't follow. Dusts are isolated particulates; milk containing TiO2 would not expose one to the particulate.
Dusts of many things can cause cancers because of the way lung tissue reacts to the dusts, without the compound being a danger in any other form.
Plus note that the reference to lung cancer was in mice, not people, and the studies on people working with TiO2 did not find it to do so in practice, so far.
(Eg. if it's in milk rather than a concentrated dust, normal coughing would dislodge it effectively, for one.) — Preceding unsigned comment added by 173.11.1.218 (talk) 20:40, 1 September 2011 (UTC)
The fact is that experiments in mice are considered as predictive for humans (laws for testing new medical substances). I do not see why these tests should not be tranferable to humans. Rather I consider it as disturbing. I refer to: <Toxicity Endpoints & Tests". AltTox.org.> "The Animal Test(s) The conventional test for carcinogenicity is the long-term rodent carcinogenicity bioassay described in Organisation for Economic Cooperation and Development (OECD) Test Guideline (TG) 451. The objective of this test is "to observe test animals for a major portion of their life span for the development of neoplastic lesions during or after exposure to various doses of a test substance by an appropriate route of administration." The study is usually conducted using two species - rats and mice of both sexes. The animals are dosed by oral, dermal, or inhalation exposures, based upon the expected type of human exposure. Dosing typically lasts around two years. Certain animal health features are monitored throughout the study, but the key assessment resides in the full pathological analysis of the animal tissues and organs when the study is terminated." Two endpoints in animal bioassays, carcinogenicity and chronic toxicity, can be combined to reduce animal use, as described in OECD TG 453. 84.175.235.161 (talk) 20:00, 25 September 2012 (UTC)
Occurrence of titanium oxide
editThis sentence from the "Occurrence" section needs revising. Can someone please help?
[3] Rutile, anatase and brookite all contain six coordinated titanium. — Preceding unsigned comment added by Trains88 (talk • contribs) 18:48, 8 December 2010 (UTC)
Health and safety
editI am extremely dubious about the claim of 70% of the volume of all pigment uses. In virtually every paint formula I have seen, titanium dioxide volume is overshadowed by filler pigments such as calcium carbonate and talc. -Tom2K — Preceding unsigned comment added by Tom2K (talk • contribs) 13:19, 21 October 2013 (UTC)
deleted this text ", suggesting that humans may be at risk of cancer or genetic disorders resulting from exposure" from the sentence "Studies have also found that titanium dioxide nanoparticles cause genetic damage in mice, suggesting that humans may be at risk of cancer or genetic disorders resulting from exposure." to add additional information. Kmrtdsc (talk) 23:12, 22 February 2011 (UTC)
Nanotubes
editDeleted this section. This is pretty specific, a whole section seems a little much. — Preceding unsigned comment added by 129.100.60.189 (talk) 15:01, 24 June 2011 (UTC)
Based on the recent research, the battery's anode part consists of innovative titanium dioxide nanotubes instead of traditionally used graphite, which speeds up chemical reactions in the battery, allowing for extremely fast charging. This information should be added about usage of nanotubes. Link: http://eandt.theiet.org/news/2014/oct/superfast-charging-li-ion.cfm — Preceding unsigned comment added by Kenorb (talk • contribs) 10:11, 15 October 2014 (UTC)
What is safety when used directly in food?
editI read the article page and this text page. Not much seems to be said about the use of titanium dioxide directly in food. I'm very interested in a new product called Daiya (www.DaiyaFoods.com). Daiya is a vegan "cheese" substitute. One of the ingredients in Daiya is titanium dioxide.
I want to know about the safety of titanium dioxide as a food product (as opposed to inhaled or used on teeth or skin/external). I don't know if it is appropriate to ask for such a thing here since I've never submitted anything to wikipedia. Just in case it is OK to do so: Any additional information added to the article in regards to the safety of this product as a food item would be appreciated. — Preceding unsigned comment added by 199.79.32.23 (talk) 16:33, 27 June 2011 (UTC)
Health and safety
editThe entire last paragraph in this section read as though it had been copied from a chemical industry press release. Because I felt that it didn't really add anything useful and because it seemed to violate the NPOV principle, I deleted the entire paragraph.Moisture (talk) 18:53, 22 July 2011 (UTC)
I am extremely dubious about the claim of 70% of the volume of all pigment uses. In virtually every paint formula I have seen, titanium dioxide volume is overshadowed by filler pigments such as calcium carbonate and talc. Tom2K (talk) 13:21, 21 October 2013 (UTC)
- Seems to me the volume refers to "the total production volume of pigments worldwide" as stated and not the 70% of the volume of paint in a can. Removing cite needed tag as misunderstanding. Vsmith (talk) 14:26, 21 October 2013 (UTC)
- Ha. Okay, think that through: where is this excess TiO2 ending up, if it isn't going into products (the "can of paint")? Anyway, there is simply no way TiO2 production worldwide is 70% of pigment production by volume or mass. Dollarwise, we have a chance - it's a lot more expensive (good ore is harder to find, the processing is a lot more intensive) than the filler pigments. Pigment grade TiO2 costs roughly 10x what calcium carbonate costs (Both vary widely depending on grade.) Even if then number were correct, that claim needs a citation. Tom2K (talk) 13:20, 22 October 2013 (UTC)
- Ah... re-added cite needed tag. Vsmith (talk) 13:59, 26 October 2013 (UTC)
- Agree with cite needed, but the claim may not be as unlikely as suggested above. I think that TiO2 is all manufactured, whereas most calcium carbonate, and all talc, is probably extracted/quarried/mined from natural rocks. So those and other cheap fillers, it may possibly be claimed, are not part of the _production_ volume of pigments, whether by mass, volume, or value.
- Gravuritas (talk) 14:56, 26 October 2013 (UTC)
- Ah... re-added cite needed tag. Vsmith (talk) 13:59, 26 October 2013 (UTC)
- Ha. Okay, think that through: where is this excess TiO2 ending up, if it isn't going into products (the "can of paint")? Anyway, there is simply no way TiO2 production worldwide is 70% of pigment production by volume or mass. Dollarwise, we have a chance - it's a lot more expensive (good ore is harder to find, the processing is a lot more intensive) than the filler pigments. Pigment grade TiO2 costs roughly 10x what calcium carbonate costs (Both vary widely depending on grade.) Even if then number were correct, that claim needs a citation. Tom2K (talk) 13:20, 22 October 2013 (UTC)
- It may depend on the definition of "pigment". If fillers are not considered pigments in the original source, then TiO2 could easily reach 70%. — Preceding unsigned comment added by 96.57.178.234 (talk) 20:20, 27 February 2014 (UTC)
Did anyone check out this claim? "The safety of the use of nano-particle sized titanium dioxide, which can penetrate the body and reach internal organs, has been criticized.[57]" The reference has no critisizing of nanoparticle research at all, it proposes a link between titanium dioxide nanoparticles and Alzheimer's.
The whole paragraph sounds like it is attempting to downplay the risks of titanium dioxide nanoparticles, and I feel that it came off as very biased. — Preceding unsigned comment added by 69.218.76.129 (talk) 04:16, 1 March 2014 (UTC)
The paragraph "Many sunscreens use nanoparticle titanium dioxide (along with nanoparticle zinc oxide) which, despite reports of potential health risks, is not actually absorbed through the skin." somewhat contradicts the related source, which states in the title that there is a "Lack of significant dermal penetration of titanium dioxide (...)", which likely means that, albeit lower than assumed relevant at said time, a certain penetration does occur.
In addition, the following may be relevant to this section:
"The study raises the possibility that humanity’s increasing use of TiO2 pigment accounts for part of the global increase in the incidence of T2D [Type 2 Diabetes]." lmaxmai (talk) 16:33, 5 July 2018 (UTC)
Opacity?
editThe article seems to suggest that titanium dioxide's apparent opacity and white color is due entirely to its high index of refraction. Is that right, or is it actually opaque as a pure bulk crystal? —Ben FrantzDale (talk) 16:45, 2 September 2011 (UTC)
- Ideally it is transparent, and the color or grainy/milky appaearance is due to various impurities and structural defects. Materialscientist (talk) 23:21, 2 September 2011 (UTC)
- That's very interesting. I had always assumed it was a fine powder of tiny opaque particles. With a citation, this would be a very interesting fact to add to the page. It is a subtlety that leads to a lot of other interesting optical facts. I found this page has a photo claiming to be of a large crystal of the stuff. —Ben FrantzDale (talk) 14:37, 6 September 2011 (UTC)
- High refractive index is certainly the major factor in opacity for paints etc:- is this a suitable source?
- [[3]]
- Gravuritas (talk) 15:03, 26 October 2013 (UTC)
- That's very interesting. I had always assumed it was a fine powder of tiny opaque particles. With a citation, this would be a very interesting fact to add to the page. It is a subtlety that leads to a lot of other interesting optical facts. I found this page has a photo claiming to be of a large crystal of the stuff. —Ben FrantzDale (talk) 14:37, 6 September 2011 (UTC)
Strange ref
editThe reference for this sentence, "Titanium dioxide is used to mark the white lines on the tennis courts of the All England Lawn Tennis and Croquet Club, best known as the venue for the annual grand slam tennis tournament The Championships, Wimbledon" has nothing to do with Wimbledon. The ref is Light spells doom for bacteria -- Mwanner | Talk 17:40, 11 March 2012 (UTC)
- It's mentioned in the article, but not that specifically. --vuo (talk) 18:20, 11 March 2012 (UTC)
- That's kind of an understatement :-) It reads "The antimicrobial paints could be the interior décor of choice for cleanrooms, child-care centers, restaurant kitchens, and public and domestic bathrooms. They contain nanoparticles of titanium dioxide, a white compound used as a brightener in commercial paints and as the powder for the bright white lines on some tennis courts." (Emphasis added)
- Where's it say anything about Wimbledon? I'm inclined to delete the Wimbledon mention, and use "some tennis courts" instead. -- Mwanner | Talk 17:22, 13 March 2012 (UTC)
- Agreed — Preceding unsigned comment added by 108.180.197.52 (talk) 01:53, 10 November 2012 (UTC)
Multi-use TiO2 nanofiber membrane from Nanyang Technological University
editSaw some info on this on TV and checked. It seems to be very important but I'm not a skilled Wiki editor myself. I wonder if it should be put it in the article. Can anyone help?
- concurrently produce both hydrogen and clean water when exposed to sunlight
- be made into a low-cost flexible filtration membrane that is anti-fouling
- desalinate water as a high flux forward osmosis membrane
- recover energy from waste desalination brine and wastewater
- be made into a low-cost flexible solar cell to generate electricity
- doubles battery life when used as anode in lithium ion battery
- kill harmful microbial, leading to new antibacterial bandages
- Main source: http://media.ntu.edu.sg/NewsReleases/Pages/newsdetail.aspx?news=14e3b618-c71c-4f20-935c-2a566af5a298
- D.D. Sun (Darren Delai Sun)'s published papers: http://www.ntu.edu.sg/home/ddsun/publication.html
More info:
- http://www.sciencedaily.com/releases/2013/03/130320094856.htm
- http://www.sciencedirect.com/science/article/pii/S0926337313001926
--KenLee318 (talk) 09:57, 27 April 2013 (UTC)
Question
editWhat's the relevance of this link in the main article to a "Timeline of hydrogen technologies" when this timeline makes no mention of Titanium at all? — Preceding unsigned comment added by 217.155.200.241 (talk) 11:40, 19 June 2015 (UTC)
- No apparent connection to this link and deleted.Ekem (talk) 13:20, 27 July 2015 (UTC)
Is Titanium Dioxide is Magnetic? — Preceding unsigned comment added by 14.96.3.128 (talk) 08:07, 17 March 2016 (UTC)
External links modified
editHello fellow Wikipedians,
I have just modified one external link on Titanium dioxide. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Corrected formatting/usage for http://nsl.caltech.edu/research.nt.html
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—cyberbot IITalk to my owner:Online 08:49, 2 April 2016 (UTC)
453380 citations and counting ...
editAccording to Chemical Abstracts, 453380 articles, reports, patents, etc describe TiO2, of these 6429 are reviews (per WP:SECONDARY, of which 1894 appeared in the preceding 5 years.--Smokefoot (talk) 13:16, 28 November 2017 (UTC)
Probably not applications
editToday I removed this well intentioned list of potential applications. Given the number of reviews, we could probably stick to them for sources.--Smokefoot (talk) 13:16, 28 November 2017 (UTC)
- Titanium dioxide in solution or suspension can be used to cleave protein that contains the amino acid proline at the site where proline is present.[1]
- Titanium oxide/titanate nanotube made by hydrothermal synthesis has hydroxyl groups which make it easily dispersed in water and can be used to increase the mechanical properties of polymer.[2] Its functional groups may also react with polymer which significantly enhance the glass transition temperature (Tg) of polymer.[3]
- Titanium dioxide is also used as a material in the memristor, a new electronic circuit element. It can be employed for solar energy conversion based on dye, polymer, or quantum dot sensitized nanocrystalline TiO2 solar cells using conjugated polymers as solid electrolytes.[4]
- Due to the significant ionic and electronic conduction of TiO2, it is potent to be used as the mixed conductor.[5]
- Synthetic single crystals and films of TiO2 are used as a semiconductor,[6] and also in Bragg-stack style dielectric mirrors owing to the high refractive index of TiO2 (2.5–2.9).[7][8]
- Both anatase and rutile TiO2 can intercalate cations, acting as anode material in Li-ion batteries[9][10] and Na-ion batteries.[11]
References
- ^ Jones, BJ; Vergne, MJ; Bunk, DM; Locascio, LE; Hayes, MA (2007). "Cleavage of Peptides and Proteins Using Light-Generated Radicals from Titanium Dioxide". Anal. Chem. 79 (4): 1327–1332. doi:10.1021/ac0613737. PMID 17297930.
- ^ Cite error: The named reference
ReferenceBwas invoked but never defined (see the help page). - ^ Cite error: The named reference
ReferenceAwas invoked but never defined (see the help page). - ^ Lewis, Nathan. "Nanocrystalline TiO2". Research. California Institute of Technology. Archived from the original on 16 April 2009. Retrieved 9 October 2009.
{{cite web}}: CS1 maint: unfit URL (link) - ^ "Mixed conductors". Max Planck institute for solid state research. Retrieved 16 September 2016.
- ^ Earle, M. D. (1942). "The Electrical Conductivity of Titanium Dioxide". Physical Review. 61 (1–2): 56. Bibcode:1942PhRv...61...56E. doi:10.1103/PhysRev.61.56.
- ^ Paschotta, Rüdiger. "Bragg Mirrors". Encyclopedia of Laser Physics and Technology. RP Photonics. Retrieved 1 May 2009.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Lindström, Henrik; Södergren, Sven; Solbrand, Anita; Rensmo, Håkan; Hjelm, Johan; Hagfeldt, Anders; Lindquist, Sten-Eric (1997-09-01). "Li+ Ion Insertion in TiO2 (Anatase). 2. Voltammetry on Nanoporous Films". The Journal of Physical Chemistry B. 101 (39): 7717–7722. doi:10.1021/jp970490q. ISSN 1520-6106.
- ^ Du, Xianfeng; Wang, Qianwen; Feng, Tianyu; Chen, Xizi; Li, Liang; Li, Long; Meng, Xiangfei; Xiong, Lilong; Sun, Xiaofei (2016-02-04). "One-step Preparation of Nanoarchitectured TiO2 on Porous Al as Integrated Anode for High-performance Lithium-ion Batteries". Scientific Reports. 6. doi:10.1038/srep20138. ISSN 2045-2322. PMC 4740746. PMID 26841711.
- ^ Su, Dawei; Dou, Shixue; Wang, Guoxiu (2015-09-08). "Anatase TiO2: Better Anode Material Than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries". Chemistry of Materials. 27 (17): 6022–6029. doi:10.1021/acs.chemmater.5b02348. ISSN 0897-4756.
Food Additive
editIs there a reason why the use of Titanium dioxide as a Food additive is excluded from the article?
- Titanium Dioxide in Food www.webmd.com
- Titanium Dioxide in Food — Should You Be Concerned? healthline.com
- Titanium dioxide: E171 no longer considered safe when used as a food additive European Food Safety Authority
// Knowsetfree (talk) 00:09, 29 October 2021 (UTC)
- More than one publication/patent appears daily on this stuff.--Smokefoot (talk) 03:21, 29 October 2021 (UTC)
- I was shocked upon learning that Titanium dioxide is actually put into some commercial processed foods. So I came to WikiPedia hoping for some unbiased truth and there is nothing. What's up with that???? Knowsetfree (talk) 00:35, 5 November 2021 (UTC)
- The fact that it's no longer considered safe to use in food doesn't mean that it's *actually* no longer used in food. I bought a pack of candy just the other day that has it listed as an ingredient. Fact of the matter is that it is still used as a food additive outside of the EU, and that should probably be noted somewhere (along with the potential concerns about its continued use in such a capacity).128.154.172.234 (talk) 15:25, 6 April 2022 (UTC)
Health and safety
editCompanies such as Mars and Dunkin' Donuts dropped titanium dioxide from their merchandise in 2015 after public pressure. Mars has not dropped titanium dioxide according to a lawsuit filed in 2022. However, Andrew Maynard, director of Risk Science Center at the University of Michigan, downplayed the supposed danger from use of titanium dioxide in food.
I take issue with the use of "downplayed", as downplayed implies that its more significant than he is letting on - and this is presented as a fact. Even if he is wrong - this is a NPOV issue.
I recommend changing to something like "argues that in his view, the risk of titanium dioxide in food is exaggerated."
"He says that the titanium dioxide used by Dunkin' Brands and many other food producers is not a new material, and it is not a nanomaterial either. Nanoparticles are typically smaller than 100 nanometres in diameter, yet most of the particles in food grade titanium dioxide are much larger. Still, size distribution analyses showed that batches of food-grade TiO₂ always include a nano-sized fraction as inevitable byproduct of the manufacturing processes."
I would prefer a direct quote to what is borderline editorializing here.
If you just want to say he's wrong, then this section should be removed altogether. If you want to provide an opposing viewpoint, then this should be more professionally worded. Otherwise this just seems like a callout. This is an encyclopedia on a compound, not an opinion piece. Everything on here should be factual and shouldn't be used simply to disprove someone with no significance to the discovery, invention or otherwise to the compound, even if they're wrong, especially if they're wrong.
Maynard states in his article for The Conversation, an independent academic news and research site, titled "Dunkin’ Donuts ditches titanium dioxide – but is it actually harmful?" the following quote: "The titanium dioxide used by Dunkin’ Brands and many other food producers is not a new material, and it’s not really a “nanomaterial” either. Nanoparticles are typically smaller than 100 nanometers in diameter. Yet most of the particles in food grade titanium dioxide are larger than this. They have to be for the powder to be of any use in food products."
Obviously this needs some work, but I would prefer to see something more with this verbiage - followed by any additional information after -
Still, size distribution analyses showed that batches of food-grade TiO₂ always include a nano-sized fraction as inevitable byproduct of the manufacturing processes.
This is directly plagiarized from its source. To have something like this follow in a short section makes it appear as if the purpose of the entire section was only to discredit him. If he is to be discredited, then he should not be mentioned and this should be removed. The purpose of wikipedia is to compile information.
Adding to this, you might want to have something after that resolves the contradiction, allowing both sides saying something along the lines of
Despite the contradiction, the relative percentage of nanoparticles was not stated in the source, so the negligibility of the nanoparticles, though harmful in significant amounts, is unknown.
DarmaniLink (talk) 12:01, 27 July 2022 (UTC)
- I'm not going to blank this, but this was when i was new. Please disregard. DarmaniLink (talk) 08:10, 23 February 2024 (UTC)
What exactly TiO2 is.
editI have extensive professional experience using TiO2 for industrial pigments. First, pigment isn't a dye. Second, pigment isn't necessarily inorganic, but most are. (There're also some metal-organics.) Third, I can only speak to its use in the USA. Fourth, "fillers" are, by definition, used because they reduce the cost of a mixture - they occupy (i.e. "fill") space which would otherwise be occupied by the more expensive components. (Fillers often also change the properties of the mixture, sometimes for the better, sometimes for the worse. They usually impact color (act as pigments), but they're inefficient pigments (which for some uses is acceptable).) To some extent, "functional filler" is an oxymoron, but in detail is often (a neglected) truth, fwiw. But enough about fillers. Rutile and Anatase are the only *crystalline* forms of TiO2 sold in large volumes. Over the past several decades "nano" forms (which may or may not be crystalline) have entered the market. This article blunders BADLY in dumping all of these DIFFERENT chemicals, with DIFFERENT CHEMICAL PROPERTIES, into the same basket. I state, categorically, that IF a designation (name) for a type of TiO2 includes more than 1 of the 3 above (Rutile, Anatase, nano) then that designation is obsolete and should be depreciated. (If I recall (I may not) correctly, in mid-20th Century USA Anatase predominated, but since Rutile has higher refractive index, less of the more expensive material is needed for the same "hiding power" and/or "whiteness". The cost/benefit equation often, but not always, favors the Rutile more recently. Last and perhaps most important. It is simply NOT TRUE that the material known as TiO2 commercially is simply TiO2. This article completely ignores the FACT that a lot of (most of the industrial pigment grades) Rutile is COATED. That is, each particle is composed of a core of TiO2 surrounded by a thin shell. I believe most of this is single shell, but multiple shells are also sold. The most common shell is SiO2 (amorphous (iirc) silica) but ZrO2 was at one time, an alternative. In addition, various silanes (organo-silicon compounds) can be used to modify various properties (also organotitanates, organozirconates, and even some hydrocarbon oils). The reason the TiO2 is coated is well-known (since it was discovered in the 1950s or 60s). Rutile (I'm not sure about Anatase) is photoactive. It absorbs UV and generates free radicals on its surface. These free radicals cause rapid decomposition of organics, especially polymers (the main component of paints and plastics). I do not know if this is also the case with food grades, cosmetic grades, or pharmaceutical grades. To sum up, this article doesn't distinguish between the actual products that are sold and used. A variety of crystal structures and surface coatings are in wide use all of which effect chemical and physical properties. Since this is OR, I refrain from changing the article. But it's widely known. The article needs to be rewritten. There's increasing evidence that (some grades of) TiO2 cause DNA damage and are likely carcinogens. Simply because it is used in personal care products (pharmaceuticals, cosmetics, food) in some countries (i.e. the good ole USA) does not necessarily mean the claim that "Titanium dioxide is safe" is true. Any blanket statements like that should be viewed with a LOT of suspicion. (And should NOT be included here, imho). (to make a blanket statement myself, LOL)71.30.91.96 (talk) 18:03, 18 May 2023 (UTC)
- @71.30.91.96 I actually read a paper about TiO2 on pub med after reading this wiki article. I basically illustrated eveything you said. I actually came to the talkpage to see if anyone had wondered why the article seemed to neglect to mention most (if not all) of what you and the paper mentioned. Just wanted to thank you 96.227.239.25 (talk) 21:36, 6 April 2024 (UTC)