Talk:Aluminium fluoride
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||||||||||||
| |||||||||||||||||||||
"sublimes without melting" is redundant. It should just say "sublimes".
Hazard Classifications
editThe Hazard Classification at german and portuguese sites are different. Also R- and S-phrases exist at german site. What is the correct classification? --217.111.110.4 (talk) 11:27, 22 August 2011 (UTC)
Incorrect Citations
editThe second citation on the page is incorrect and needs to be redirected to a better source. —Preceding unsigned comment added by 97.76.15.204 (talk) 17:52, 17 May 2009 (UTC)
Contradictions
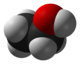
editThe text says "polymeric structure" but the image shows a single covalent molecule. The melting or sublimation points also need to be straightened out. —Keenan Pepper 15:52, 13 July 2006 (UTC)
I have never heard of AlF3 being polymeric but i don't see why not (except the nonpolar part...) --- How is this contradictary though —The preceding unsigned comment was added by Frozenport (talk • contribs) .
- How is it contradictory? The text says it's polymeric, but the picture doesn't show a polymer. I don't know how else to explain it... —Keenan Pepper 04:57, 14 July 2006 (UTC)
- I suggest you contact the author of the pic, which looks like it was done in Paintbrush (sorry)... 68.39.174.238 09:22, 15 July 2006 (UTC)
The solid is polymeric, but the picture is depicting the four-coordinate gas-phase monomeric form. Perhaps it needs a label/description? Shred-69 (talk) 04:28, 12 February 2008 (UTC)
production
editthe wording is ambiguous too ambiguous.What do i want to... (talk) 13:06, 15 December 2009 (UTC)What do i want to...
Fluoride / Trifluoride
editThe NIST chemistry webbook calls AlF3 aluminum trifluoride (it has gas phase data which are obviously not from a polymeric state) and calls Al2F6 aluminum fluoride (it has got gas phase data about that substance, too) . Icek 22:08, 12 May 2007 (UTC)
As +3 is the usual oxidation state of aluminium/aluminum, using the term "fluoride" would be acceptable in all but the most formal terms or publications. As this is an encyclopedia, and hence formal, the title should read "Aluminum (III) fluoride" as per the equivalent gallium page, or "Aluminum trifluoride". I'm in the process of building the gallium fluoride page at the moment. Shred-69 (talk) 06:39, 11 February 2008 (UTC)
- Articles are not given the most formal name possible! The general rule is that articles should adopt the most common name for the compound.
- without disagreeing with the above discussion, i suggest that while the title remains Aluminium fluoride, the more formal/"most correct" name Aluminium trifluoride (Aluminum (III) fluoride) should be also mentioned in the lede with a short explanation attached, like "As +3 is the usual oxidation state of aluminium/aluminum, using the term "fluoride" is generally accepted though according to the naming conventions it is correctly Aluminium trifluoride (Aluminum (III) fluoride)". Or something along this line.89.134.199.32 (talk) 21:58, 30 January 2019 (UTC).
niche uses
edittheres a toothpaste brand "Lacalut" that lists Aluminium fluoride among its components though it is unclear whether this is an "active ingredient" ie.: if it is there to propagate fluoridation of the teeth or just for fancy(like marketing reasons, taste, abrasive, etc). from the article information it is not clearly decideable whether such use of AlF3 is likely or not. please if you expand this article one day, keep in mind this question. 89.134.199.32 (talk) 22:09, 30 January 2019 (UTC).
"AlF" listed at Redirects for discussion
editAn editor has identified a potential problem with the redirect AlF and has thus listed it for discussion. This discussion will occur at Wikipedia:Redirects for discussion/Log/2022 May 21#AlF until a consensus is reached, and readers of this page are welcome to contribute to the discussion. 1234qwer1234qwer4 13:10, 21 May 2022 (UTC)