Sodium fluorosilicate is a compound with the chemical formula Na2[SiF6]. Unlike other sodium salts, it has a low solubility in water.
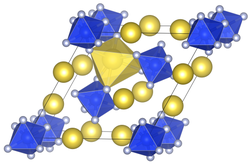 Unit cell of sodium hexafluoridosilicate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium fluorosilicate | |
| Systematic IUPAC name
Sodium hexafluoridosilicate(2–)
[1] | |
| Other names
Disodium hexafluorosilicate/sodium fluosilicate/sodium silicofluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.198 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2674 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na2[SiF6] | |
| Molar mass | 188 g/mol |
| Appearance | white granular powder |
| Odor | odorless |
| Density | 2.7 g/cm3 |
| 0.64 g/100 mL (20 °C) 1.27 g/100 mL (50 °C) 2.45 g/100 mL (100 °C) | |
| Solubility | insoluble in alcohol |
Refractive index (nD)
|
1.312 |
| Structure[2] | |
| trigonal | |
| P321 | |
a = 8.859, c = 5.038
| |
Formula units (Z)
|
4 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
70 mg/kg (mouse, oral) 125 mg/kg (rabbit, oral)[3] |
| Related compounds | |
Other cations
|
Ammonium hexafluorosilicate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Natural occurrence
editSodium hexafluorosilicate occurs naturally as the rare mineral malladrite found within some volcanic fumaroles.[4]
Manufacturing
editSodium fluorosilicate is made by neutralizing fluorosilicic acid with sodium chloride or sodium sulfate.
- H2[SiF6] + 2 NaCl → Na2[SiF6] + 2 HCl
Possible application
editSodium fluorosilicate is used in some countries as additives for water fluoridation, opal glass raw material, ore refining, or other fluoride chemical (like sodium fluoride, magnesium silicofluoride, cryolite, aluminum fluoride) production.[5]
It also is an ingredient in some ceramic cements.
See also
editReferences
edit- ^ "Parent Hydride Names and Substitutive Nomenclature". Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 (PDF). RSC Publishing. 2005. pp. 114–135.
- ^ Allan Zalkin, J. D. Forrester, David H. Templeton (1964). "The Crystal Structure of Sodium Fluorosilicate". Acta Crystallographica. 17 (11): 1408–1412. Bibcode:1964AcCry..17.1408Z. doi:10.1107/S0365110X64003516.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Fluorides (as F)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Malladrite".
- ^ "PUB". Archived from the original on 2009-03-26. Retrieved 2009-08-10.
