Salicylanilide is a chemical compound which is the amide of salicylic acid and aniline. It is classified as both a salicylamide and an anilide.[3]

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxy-N-phenylbenzamide | |
| Other names
2-Hydroxybenzanilide
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1108135 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.571 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H11NO2 | |
| Molar mass | 213.236 g·mol−1 |
| Appearance | White to off-white crystalline solid |
| Melting point | 136 to 138 °C (277 to 280 °F; 409 to 411 K) |
| Hazards | |
| GHS labelling:[2] | |
 
| |
| Warning | |
| H315, H319, H335, H400 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
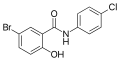
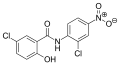
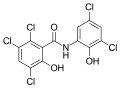
Derivatives of salicylanilide have a variety of pharmacological uses. Chlorinated derivatives including niclosamide, oxyclozanide, and rafoxanide are used as anthelmintics, especially as flukicides. Brominated derivatives including dibromsalan, metabromsalan, and tribromsalan are used as topical antibacterials and antifungals.
-
Niclosamide
-
Oxyclozanide
-
Rafoxanide
References
edit- ^ Salicylanilide at chemicalland21.com
- ^ "Salicylanilide". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- ^ Salicylanilides at the U.S. National Library of Medicine Medical Subject Headings (MeSH)