This article relies largely or entirely on a single source. (July 2021) |
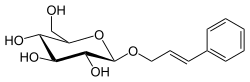
Rosin is a glycoside ester of cinnamyl alcohol and a constituent of Rhodiola rosea.
| |
| Names | |
|---|---|
| IUPAC name
(2E)-3-Phenylprop-2-en-1-yl β-D-glucopyranoside
| |
| Systematic IUPAC name
(2R,3S,4S,5R,6R)-2-(Hydroxymethyl)-6-{[(2E)-3-phenylprop-2-en-1-yl]oxy}oxane-3,4,5-triol | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H20O6 | |
| Molar mass | 296.32 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Related compounds
editThe three cinnamyl alcohol-vicianosides of Rhodiola rosea, commonly referred to as "rosavins," are rosin, and the structurally related disaccharide rosavin, which is the arabinose ester of rosin, and rosarin, the arabinofuranose ester of rosin. Salidroside, common in Rhodiola spp. and occurring in Rhodiola rosea is not a cinnamyl alcohol glycoside, but a glycoside of tyrosol.[1]
Sources
editThe cinnamyl alcohol glycosides rosin, rosavin and rosarin occur in the context of rhodiola species, only in Rhodiola rosea.[1]
References
edit- ^ a b György, Zsuzsanna (2006-05-22). "Glycoside production by in vitro Rhodiola rosea cultures" (PDF). Acta Universitatis Ouluensis C Technica. 244. University of Oulu. ISBN 951-42-8080-6. ISSN 1796-2226.