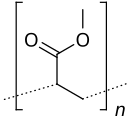
Poly(methyl acrylate) (PMA) is a family of organic polymers with the formula (CH2CHCO2CH3)n. It is a synthetic acrylate polymer derived from methyl acrylate monomer. The polymers are colorless. This homopolymer is far less important than copolymers derived from methyl acrylate and other monomers. PMA is softer than polymethyl methacrylate (PMMA),[1] It is tough, leathery, and flexible.[2]
| |
| Names | |
|---|---|
| Other names
Polymethylacrylate
Polymethyl acrylate | |
| Identifiers | |
| Abbreviations | PMA |
| ChEBI | |
| ChemSpider |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C4H6O2)n | |
| Appearance | colorless solid |
| Density | 1.20 g/cm3 |
Refractive index (nD)
|
1.479 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copolymers
editFar more important than PMA are copolymers produced from methyl acrylate and one or more of the following comonomers methyl methacrylate, styrene, acrylonitrile, vinyl acetate, vinyl chloride, vinylidene chloride, and butadiene.[3]
Properties
editIt has a low glass-transition temperature about 10 °C (12.5 °C in case of PMA38).[4] It is soluble in dimethyl sulfoxide (DMSO).[4] PMA is water-sensitive and unlike PMMA, is not stable against alkalies.[2]
High-energy radiation leads to cross linking in PMA. However in polymethyl methacrylate (PMMA), a compound similar to PMA, degradation occurs instead.[1]
Uses
editAlso used in leather finishing and textiles.[2]
Derivatives of this polymer are commonly used in orally administerd pharmaceutical formulations to target specific regions of the gastrointestinal tract.[5]
References
edit- ^ a b Peter A. Ciullo (1996). Industrial Minerals and Their Uses: A Handbook and Formulary. Elsevier. p. 115. ISBN 978-0-8155-1408-4. Retrieved 30 June 2012.
- ^ a b c J. A. Brydson (8 November 1999). Plastics Materials. Butterworth-Heinemann. p. 423. ISBN 978-0-7506-4132-6. Retrieved 30 June 2012.
- ^ Penzel, Erich; Ballard, Nicholas; Asua, José M. (2018). "Polyacrylates". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–20. doi:10.1002/14356007.a21_157.pub2. ISBN 9783527306732.
- ^ a b Kyle B. Guice (2008). Synthesis & Characterization of Temperature- and PH-responsive Nanostructures Derived from Block Copolymers Containing Statistical Copolymers of HEMA and DMAEMA. p. 29. ISBN 978-0-549-63651-9. Retrieved 30 June 2012.
- ^ "Eudragit a versatile polymer: A review".