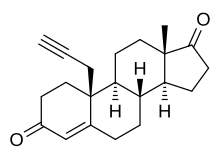
Plomestane (INN, USAN; former developmental code name MDL-18962; also known as propargylestrenedione, PED) is a steroidal, irreversible aromatase inhibitor which was under development by Marion Merrell Dow/Hoechst Marion Russell (now Hoechst AG) as an antineoplastic agent for the treatment of breast cancer.[1][2][3][4][5] It was found to be effective in preclinical studies and was also found to produce few adverse effects in human clinical trials, significantly reducing estrogen levels with a single administration.[5] However, development of the drug for clinical use was halted due to "technical issues" and it was never marketed.[6]
 | |
| Clinical data | |
|---|---|
| Other names | MDL-18962; Propargylestrenedione; PED; 10-(2-Propyn-1-yl)estr-4-ene-3,17-dione; 10-Propargylestr-4-ene-3,17-dione |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H26O2 |
| Molar mass | 310.437 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
In addition to its activity as an aromatase inhibitor, plomestane has weak androgenic properties.[5]
See also
editReferences
edit- ^ Macdonald F (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1635. ISBN 978-0-412-46630-4. Retrieved 19 May 2012.
- ^ Morton IK, Hall JM (1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer. p. 227. ISBN 978-0-7514-0499-9. Retrieved 20 May 2012.
- ^ Lombardi P (June 1995). "The irreversible inhibition of aromatase (oestrogen synthetase) by steroidal compounds". Current Pharmaceutical Design. 1. Bentham Science Publishers: 23–50 (45). doi:10.2174/1381612801666220524190226. S2CID 249298105. Retrieved 20 May 2012.
- ^ Kreider RB, Leutholtz BC, Katch FI, Katch VL (2009). Exercise and Sport Nutrition. Exercise & Sport Nutrition. p. 350. ISBN 978-0-9742965-6-2. Retrieved 20 May 2012.
- ^ a b c Kelloff GJ, Lubet RA, Lieberman R, et al. (January 1998). "Aromatase inhibitors as potential cancer chemopreventives". Cancer Epidemiology, Biomarkers & Prevention. 7 (1): 65–78. PMID 9456245.
- ^ Avendaño C, Menéndez JC (4 June 2008). Medicinal Chemistry of Anticancer Drugs. Elsevier. p. 69. ISBN 978-0-444-52824-7. Retrieved 20 May 2012.