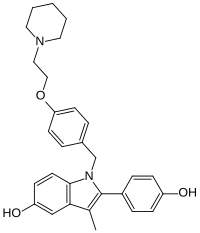
Pipendoxifene (INN) (developmental code name ERA-923) is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development by Ligand Pharmaceuticals and Wyeth-Ayerst Laboratories (now Wyeth) for the treatment of breast cancer but was not marketed.[1][2][3] It is a member of the 2-phenylindole group of SERMs and is structurally related to zindoxifene and the marketed bazedoxifene.[2][3] The drug reached phase II clinical trials before its development was discontinued.[1][2] It was synthesized at the same time as bazedoxifene and was intended as a backup drug for bazedoxifene, only to be developed further if bazedoxifene had failed in clinical trials.[1][2] No further development was reported after 2002 and it was formally announced that development had been terminated in November 2005.[1][4]
 | |
| Clinical data | |
|---|---|
| Other names | ERA-923 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H32N2O3 |
| Molar mass | 456.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Unlike the SERM raloxifene, pipendoxifene is devoid of uterotrophic activity in immature/ovariectomized rodents.[3][5]
References
edit- ^ a b c d "Pipendoxifene". Addis Insight. Springer Nature Switzerland AG.
- ^ a b c d Gribble GW (9 October 2010). Heterocyclic Scaffolds II:: Reactions and Applications of Indoles. Springer Science & Business Media. pp. 14–. ISBN 978-3-642-15732-5.
- ^ a b c Prudhomme M (14 June 2013). Advances in Anticancer Agents in Medicinal Chemistry. Bentham Science Publishers. pp. 368–369. ISBN 978-1-60805-496-1.
- ^ Ottow E, Weinmann H (8 September 2008). Nuclear Receptors as Drug Targets. John Wiley & Sons. pp. 95–. ISBN 978-3-527-62330-3.
- ^ Cano A, Calaf i Alsina J, Duenas-Diez JL (22 September 2006). Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs. Springer Science & Business Media. pp. 58–. ISBN 978-3-540-34742-2.
External links
edit