Phenbenzamine, sold under the brand name Antergan and known by the former developmental code name RP-2339, is an antihistamine of the ethylenediamine class which also has anticholinergic properties.[1][2] It was introduced in 1941 or 1942 and was the first antihistamine to be introduced for medical use.[3][4][5] Soon following its introduction, phenbenzamine was replaced by another antihistamine of the same class known as mepyramine (pyrilamine; Neoantergan).[5][6] Following this, other antihistamines, such as diphenhydramine, promethazine, and tripelennamine, were developed and introduced.[5][7] Owing to their sedative effects, phenbenzamine and promethazine were assessed in the treatment of manic depression in France in the 1940s and were regarded as promising therapies for such purposes.[3] Whereas phenbenzamine was the first clinically useful antihistamine, piperoxan was the first compound with antihistamine properties to be discovered and was synthesized in the early 1930s.[7]
 | |
| Clinical data | |
|---|---|
| Trade names | Antergan |
| Other names | RP-2339 |
| Drug class | Antihistamine; H1 receptor antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
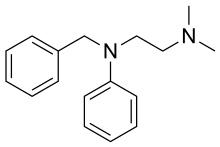
| Formula | C17H22N2 |
| Molar mass | 254.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chemistry
editSynthesis
editPhenbenzamine can be prepared by the reaction of N-benzylaniline with 2-chloroethyldimethylamine.[8][9]
References
edit- ^ "Phenbenzamine". Encyclopædia Britannica.
- ^ Maxwell RA, Eckhardt SB (6 December 2012). "Chloropromazine". Drug Discovery: A Casebook and Analysis. Springer Science & Business Media. pp. 113–. ISBN 978-1-4612-0469-5.
- ^ a b Moncrieff, Joanna (2013). "Chlorpromazine: The First Wonder Drug". The Bitterest Pills. Palgrave Macmillan UK. pp. 20–38. doi:10.1057/9781137277442_2. ISBN 978-1-137-27743-5.
- ^ Williams DA, Foye WO, Lemke TL (2002). "Chapter 29: Estrogen, Progestins, and Androgens". Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 799–. ISBN 978-0-683-30737-5.
- ^ a b c Welcome MO (20 June 2018). Gastrointestinal Physiology: Development, Principles and Mechanisms of Regulation. Springer. pp. 827–. ISBN 978-3-319-91056-7.
- ^ Cundell DR, Mickle KE (11 July 2018). "Developing the Perfect Antihistamine for use in Allergic Conditions: A Voyage in H1 Selectivity". In Atta-ur-Rahman (ed.). Frontiers in Clinical Drug Research - Anti-Allergy Agents. Bentham Science Publishers. pp. 29–. ISBN 978-1-68108-337-7.
- ^ a b Landau R, Achilladelis B, Scriabine A (1999). Pharmaceutical Innovation: Revolutionizing Human Health. Chemical Heritage Foundation. pp. 230–231. ISBN 978-0-941901-21-5.
- ^ US 2634293, Kyrides LP, Zienty FB, "Process of preparing a monobasic salt of a secondary amine", issued 7 April 1953, assigned to Monsanto Chemicals
- ^ Kaye IA, Parris CL, Weiner N (1953). "A Novel N-Alkylation Reaction". Journal of the American Chemical Society. 75 (3): 744–745. doi:10.1021/ja01099a508.