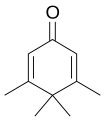
Penguinone is an organic compound with the molecular formula C
10H
14O. Its name comes from the fact that its 2-dimensional molecular structure resembles a penguin.[1][2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3,4,4,5-Tetramethylcyclohexa-2,5-dien-1-one | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H14O | |||
| Molar mass | 150.221 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
The suffix "-one" indicates that it is a ketone.[3] The systematic name of the molecule is 3,4,4,5-tetramethylcyclohexa-2,5-dienone.[4][5]
Although it is a dienone and thus has the necessary structure for a dienone–phenol rearrangement, the methyl groups in positions 3 and 5 of the ring block the movement of the group at position 4, so even the action of trifluoroacetic acid will not cause transformation to a phenol.[6]
See also
editReferences
edit- ^ May, Paul (2008). Molecules with Silly or Unusual Names. Imperial College London. p. 35. ISBN 978-1848162075.
- ^ May, Paul (23 October 2014). "Molecules with Silly or Unusual names". University of Bristol. Retrieved 1 December 2014.
- ^ Laszlo, Pierre (2004). "Science as Play". American Scientist. 92 (5): 398. doi:10.1511/2004.5.398. Archived from the original on 2016-10-18. Retrieved 2014-12-01.
- ^ "Chemical structures beginning with P". about.com. Archived from the original on 21 December 2009. Retrieved 1 December 2014.
- ^ Parkvall, Mikael (2006). Limits of Language. London: Battlebridge. p. 176. ISBN 1903292042.
- ^ Hagenbruch, Bernd; Hünig, Siegfried (1983). "Ein Beitrag zur Dienon-Phenol-Umlagerung". Chemische Berichte (in German). 116 (12): 3884––3894. doi:10.1002/cber.19831161212.