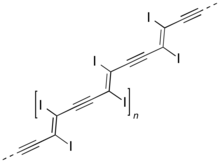
PIDA, or poly(diiododiacetylene), is an organic polymer that has a polydiacetylene backbone. It is one of the simplest polydiacetylenes that has been synthesized, having only iodine atoms as side chains. It is created by 1,4 topochemical polymerization of diiodobutadiyne.[2] It has many implications in the field of polymer chemistry as it can be viewed as a precursor to other polydiacetylenes by replacing iodine atoms with other side chains using organic synthesis, or as an iodinated form of the carbon allotrope carbyne.
| |
| Names | |
|---|---|
| IUPAC name
Poly(diiododiacetylene)
| |
Other names
| |
| Identifiers | |
| ChemSpider |
|
| Properties | |
| Appearance | Dark reflective solid |
| Insoluble, forms aggregates | |
| Explosive data | |
| Shock sensitivity | Sensitive - may explode if struck[1] |
| Related compounds | |
Related
|
Polydiacetylene, Diiodobutadiyne (monomer) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
editThe backbone of PIDA is highly conjugated and allows for the formation of an extended pi system along the length of the polymer. This property of PIDA allows it to transport electricity and act as a molecular wire or an organic semiconductor.[3] Considering PIDA's backbone and the fact that Iodine atoms can easily undergo elimination, it is conceivable that PIDA can be subjected to full reductive deiodination in the presence of a Lewis base, such as pyrrolidine[1] to yield carbyne.
Synthesis
editPIDA is synthesized from diiodobutadiyne via 1,4 topochemical polymerization.[2]
In order to meet the geometric requirements for polymerization, a host–guest strategy is used by combining a host molecule and diiodobutadiyne in solution and allowing co-crystallization to occur. This can be utilized because hosts that are most commonly used are able to bond to the diyne monomer by halogen bonding from the lewis acidic iodine atom to a lewis basic nitrogen of the host (usually a nitrile or pyridine). In order to give a proper repeat distance to the monomers (5 Å), the hosts also contain oxalamide groups that create a hydrogen bonding network throughout the crystal.
In most instances, polymerization is spontaneous upon crystallization or exposure to UV radiation/pressure.[2]
Reactions
editPIDA Can undergo carbonization at high temperatures near 900 °C[4] and reductive dehalogenation carbonization when exposed to pyrrolidine at room temperature.[1]
Attempts have been made to replace iodine side groups with other functional groups. There are also attempts being made at making other halogen analogs of PIDA.
See also
editReferences
edit- ^ a b c Luo, Liang; Resch, Daniel; Wilhelm, Christopher; Young, Christopher N.; Halada, Gary P.; Gambino, Richard J.; Grey, Clare P.; Goroff, Nancy S. (2011), "Room-Temperature Carbonization of Poly(diiododiacetylene) by Reaction with Lewis Bases", Journal of the American Chemical Society, 133 (48): 19274–19277, doi:10.1021/ja2073752, PMID 22035062
- ^ a b c Sun, A.; Lauher, J.W.; Goroff, N.S. (2006), "Preparation of Poly(Diiododiacetylene), an Ordered Conjugated Polymer of Carbon and Iodine", Science, 312 (5776): 1030–1034, Bibcode:2006Sci...312.1030S, doi:10.1126/science.1124621, PMID 16709780, S2CID 36045120
- ^ Luo, Liang; Wilhelm, Christopher; Sun, Aiwu; Grey, Clare P.; Lauher, Joseph W.; Goroff, Nancy S. (2008), "Poly(Diiododiacetylene): Preparation, Isolation, and Full Characterization of a Very Simple Poly(diacetylene)", Journal of the American Chemical Society, 130 (24): 7702–7709, doi:10.1021/ja8011403, PMID 18489101
- ^ Luo, Liang; Wilhelm, Christopher; Young, Christopher N.; Grey, Clare P.; Halada, Gary P.; Xiao, Kai; Ivanov, Ilia N.; Howe, Jane Y.; Geohegan, David B.; Goroff, Nancy S. (2011), "Characterization and Carbonization of Highly Oriented Poly(diiododiacetylene) Nanofibers", Macromolecules, 44 (8): 2626–2631, Bibcode:2011MaMol..44.2626L, doi:10.1021/ma102324r