A nuclear clock or nuclear optical clock is an atomic clock being developed that will use the energy of a nuclear isomeric transition as its reference frequency,[1] instead of the atomic electron transition energy used by conventional atomic clocks. Such a clock is expected to be more accurate than the best current atomic clocks by a factor of about 10, with an achievable accuracy approaching the 10−19 level.[2]
| Nuclear Clock | |
|---|---|
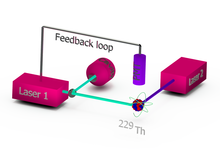 Concept of a thorium-229 based nuclear optical clock. | |
| Industry | scientific, satellite navigation, and data transfer |
| Application | time-keeping |
The only nuclear state suitable for the development of a nuclear clock using existing technology is thorium-229m, an isomer of thorium-229 and the lowest-energy nuclear isomer known. With an energy of 8.355733554021(8) eV,[3][4][5] this corresponds to a frequency of 2020407384335±2 kHz,[6] or wavelength of 148.382182883 nm, in the vacuum ultraviolet region, making it accessible to laser excitation.[7][8]
Principle of operation
editAtomic clocks are today's most accurate time-keeping devices. Their underlying principle of operation is based on the fact that the difference between two electron energy levels is independent of space and time. An atomic electron transition can be excited with electromagnetic radiation, if the radiation's photon energy exactly matches the energy of the transition. This corresponds (via the Planck relation) to a particular frequency. A source's frequency can be stabilized to match the corresponding atomic electron transition energy by continuous verification of a successful excitation of the atomic transition. Once thus stabilized, the frequency of the radiation will always be the same (independent of space and time).
Conventional atomic clocks use microwave (high-frequency radio wave) frequencies, but development of the laser has made it possible to generate very stable light frequencies, and the frequency comb makes it possible to count those oscillations (measured in hundreds of THz, trillions of cycles per second) to extraordinarily high accuracy. When the frequency of the laser has been stabilized to a particular atomic transition, the result is a measurement of time. Such a device is known as an optical atomic clock.[9]
One prominent example of an optical atomic clock is the ytterbium (Yb) lattice clock, where a particular electron transition in the ytterbium-171 isotope is used for laser stabilization.[10] In this case, one second has elapsed after 518295836590863.63±0.1 oscillations of the laser light stabilized to the corresponding electron transition.[11] Other examples for optical atomic clocks of the highest accuracy are the Yb-171 single-ion clock,[12] the strontium(Sr)-87 optical lattice clock,[13][14] and the aluminum(Al)-27 single-ion clock.[15] The achieved accuracies of these clocks vary around 10−18, corresponding to about 1 second of inaccuracy in 30 billion years, significantly longer than the age of the universe.
A nuclear optical clock would use the same principle of operation, with the important difference that a nuclear transition instead of an atomic shell electron transition is used for laser stabilization.[1] The expected advantage of a nuclear clock is that the atomic nucleus is smaller than the atomic shell by up to five orders of magnitude, with correspondingly smaller magnetic dipole and electric quadrupole moments, and is therefore significantly less affected by external magnetic and electric fields. Such external perturbations are the limiting factor for the achieved accuracies of electron-based atomic clocks. Due to this conceptual advantage, a nuclear optical clock is expected to achieve a time accuracy approaching 10−19, a ten-fold improvement over electron-based clocks.[2]
Ionization
editAn excited atomic nucleus can shed its excess energy by two alternative paths:
- radiatively, by direct photon (gamma ray) emission, or
- by internal conversion, transferring the energy to a shell electron which is ejected from the atom.
For most nuclear isomers, the available energy is sufficient to eject any electron, and the inner-shell electrons are the most frequently ejected. In the special case of 229m
Th
, the energy is sufficient only to eject an outer electron (thorium's first ionization energy is 6.3 eV), and if the atom is already ionized, there is not enough energy to eject a second (thorium's second ionization energy is 11.5 eV).
The two decay paths have different half-lives. Neutral 229m
Th
decays almost exclusively by internal conversion, with a half-life of 7±1 μs.[16] In thorium cations, internal conversion is energetically prohibited, and 229m
Th+
is forced to take the slower path, decaying radiatively with a half-life of around half an hour.[4]
Thus, in the typical case that the clock is designed to measure radiated photons, it is necessary to hold the thorium in an ionized state. This can be done in an ion trap, or by embedding it in an ionic crystal with a band gap greater than the transition energy.[17] In this case, the atoms are not 100% ionized, and a small amount of internal conversion is possible (reducing the half-life to approximately 10 minutes[4]), but the loss is tolerable.
Different nuclear clock concepts
editTwo different concepts for nuclear optical clocks have been discussed in the literature: trap-based nuclear clocks and solid-state nuclear clocks.
Trap-based nuclear clocks
editFor a trap-based nuclear clock either a single 229Th3+ ion is trapped in a Paul trap, known as the single-ion nuclear clock,[1][2] or a chain of multiple ions is trapped, considered as the multiple-ion nuclear clock.[7] Such clocks are expected to achieve the highest time accuracy, as the ions are to a large extent isolated from their environment. A multiple-ion nuclear clock could have a significant advantage over the single-ion nuclear clock in terms of stability performance.
Solid-state nuclear clocks
editAs the nucleus is largely unaffected by the atomic shell, it is also intriguing to embed many nuclei into a crystal lattice environment. This concept is known as the crystal-lattice nuclear clock.[1] Due to the high density of embedded nuclei of up to 1018 per cm3, this concept would allow irradiating a huge number of nuclei in parallel, thereby drastically increasing the achievable signal-to-noise ratio,[18] but at the cost of potentially higher external perturbations.[19] It has also been proposed to irradiate a metallic 229Th surface and to probe the isomer's excitation in the internal conversion channel, which is known as the internal-conversion nuclear clock.[20] Both types of solid-state nuclear clocks were shown to offer the potential for comparable performance.
Transition requirements
editFrom the principle of operation of a nuclear optical clock, it is evident that direct laser excitation of a nuclear state is a central requirement for the development of such a clock. This is impossible for most nuclear transitions, as the typical energy range of nuclear transitions (keV to MeV) is orders of magnitude above the maximum energy which is accessible with significant intensity by today's narrow-bandwidth laser technology (a few eV). There are only two nuclear excited states known which possess a sufficiently low excitation energy (below 100 eV). These are
- 229m
Th
, a metastable nuclear excited state of the isotope thorium-229 with an excitation energy of only about 8 eV,[21][22] and - 235m1
U
, a metastable excited state of uranium-235 with an energy of 76.7 eV.[23]
However, 235m1
U
has such an extraordinarily long radiative half-life (on the order of 1022 s, 20,000 times the age of the universe, and far longer than its internal conversion half-life of 26 minutes) that it is not practical to use for a clock.[24][25] This leaves only 229mTh with a realistic chance of direct nuclear laser excitation.
Further requirements for the development of a nuclear clock are that
- the lifetime of the nuclear excited state is relatively long, thereby leading to a resonance of narrow bandwidth (a high quality factor) and
- the ground-state nucleus is easily available and sufficiently long-lived to allow one to work with moderate quantities of the material.
Fortunately, with 229m
Th+
having a radiative half-life (time to decay to 229
Th+
) of around 103 s,[4][26][27] and 229
Th
having a half-life (time to decay to 225
Ra
) of 7917±48 years,[28] both conditions are fulfilled for 229m
Th+
, making it an ideal candidate for the development of a nuclear clock.
History
editHistory of nuclear clocks
editAs early as 1996 it was proposed by Eugene V. Tkalya to use the nuclear excitation as a "highly stable source of light for metrology".[29]
With the development (around 2000) of the frequency comb for measuring optical frequencies exactly, a nuclear optical clock based on 229m
Th
was first proposed in 2003 by Ekkehard Peik and Christian Tamm, who developed an idea of Uwe Sterr.[1] The paper contains both concepts, the single-ion nuclear clock, as well as the solid-state nuclear clock.
In their pioneering work, Peik and Tamm proposed to use individual laser-cooled 229
Th3+
ions in a Paul trap to perform nuclear laser spectroscopy.[1] Here the 3+ charge state is advantageous, as it possesses a shell structure suitable for direct laser cooling. It was further proposed to excite an electronic shell state, to achieve 'good' quantum numbers of the total system of the shell plus nucleus that will lead to a reduction of the influence induced by external perturbing fields. A central idea is to probe the successful laser excitation of the nuclear state via the hyperfine-structure shift induced into the electronic shell due to the different nuclear spins of ground- and excited state. This method is known as the double-resonance method.
The expected performance of a single-ion nuclear clock was further investigated in 2012 by Corey Campbell et al. with the result that a systematic frequency uncertainty (accuracy) of the clock of 1.5×10−19 could be achieved, which would be by about an order of magnitude better than the accuracy achieved by the best optical atomic clocks today.[2] The nuclear clock approach proposed by Campbell et al. slightly differs from the original one proposed by Peik and Tamm. Instead of exciting an electronic shell state in order to obtain the highest insensitivity against external perturbing fields, the nuclear clock proposed by Campbell et al. uses a stretched pair of nuclear hyperfine states in the electronic ground-state configuration, which appears to be advantageous in terms of the achievable quality factor and an improved suppression of the quadratic Zeeman shift.
In 2010, Eugene V. Tkalya showed that it was theoretically possible to use 229m
Th
as a lasing medium to generate an ultraviolet laser.[30][31][32]
The solid-state nuclear clock approach was further developed in 2010 by W.G. Rellergert et al.[19] with the result of an expected long-term accuracy of about 2×10−16. Although expected to be less accurate than the single-ion nuclear clock approach due to line-broadening effects and temperature shifts in the crystal lattice environment, this approach may have advantages in terms of compactness, robustness and power consumption. The expected stability performance was investigated by G. Kazakov et al. in 2012.[18] In 2020, the development of an internal conversion nuclear clock was proposed.[20]
Important steps on the road towards a nuclear clock include the successful direct laser cooling of 229
Th3+
ions in a Paul trap achieved in 2011,[33] and a first detection of the isomer-induced hyperfine-structure shift, enabling the double-resonance method to probe a successful nuclear excitation in 2018.[34]
History of 229mTh
editSince 1976, the 229Th nucleus has been known to possess a low energy excited state,[35] whose excitation energy was originally shown to less than 100 eV,[8] and then shown to be less than 10 eV in 1990.[36]
This was, however, too broad an energy range to apply high-resolution spectroscopy techniques; the transition energy had to be narrowed down first. Initial efforts used the fact that, after the alpha decay of 233
U
, the resultant 229
Th
nucleus is in an excited state and promptly emits a gamma ray to decay to either the base state or the metastable state. Measuring the small difference in the gamma-ray energies emitted in these processes allows the metastable state energy to be found by subtraction.[36][7]: §5.1 [37]: §2.3 However, nuclear experiments are not capable of finely measuring the difference in frequency between two high gamma-ray energies, so other experiments were needed.[8] Because of the natural radioactive decay of 229Th nuclei, a tightly concentrated laser frequency was required to excite enough nuclei in an experiment to outcompete the background radiation and give a more accurate measurement of the excitation energy.[8] Because it was infeasible to scan the entire 100eV range, an estimate of the correct frequency was needed.[8]
An early mis-step was the (incorrect) measurement of the energy value as 3.5±1.0 eV in 1994.[38] This frequency of light is relatively easy to work with, so many direct detection experiments were attempted which had no hope of success because they were built of materials opaque to photons at the true, higher, energy.[7][8] In particular:
- thorium oxide is transparent to 3.5 eV photons, but opaque at 8.3 eV,
- common optical lens and window materials such as fused quartz are opaque at energies above 8 eV,[39]
- molecular oxygen (air) is opaque to photons above 6.2 eV; experiments must be conducted in a nitrogen or argon atmosphere, and
- the ionization energy of thorium is 6.3 eV so the nuclei will decay by internal conversion unless prevented (see § Ionization).
The energy value remained elusive until 2003, when the nuclear clock proposal triggered a multitude of experimental efforts to pin down the excited state's parameters like energy and half-life. The detection of light emitted in the direct decay of 229m
Th
would significantly help to determine its energy to higher precision, but all efforts to observe the light emitted in the decay of 229m
Th
were failing.[7] The energy level was corrected to 7.6±0.5 eV in 2007[40] (slightly revised to 7.8±0.5 eV in 2009[41]). Subsequent experiments continued to fail to observe any signal of light emitted in the direct decay, leading people to suspect the existence of a strong non-radiative decay channel.[42][43][37][44] The detection of light emitted by the decay of 229mTh was reported in 2012,[45] and again in 2018,[46] but the observed signals were the subject of controversy within the community.[47]
A direct detection of electrons emitted by the isomer's internal conversion decay channel was achieved in 2016.[48] This detection laid the foundation for the determination of the 229mTh half-life in neutral, surface-bound atoms in 2017[16] and a first laser-spectroscopic characterization in 2018.[34]
In 2019, the isomer's energy was measured via the detection of internal conversion electrons emitted in its direct ground-state decay to 8.28±0.17 eV.[21] Also a first successful excitation of the 29 keV nuclear excited state of 229
Th
via synchrotron radiation was reported,[49] enabling a clock transition energy measurement of 8.30±0.92 eV.[50] In 2020, an energy of 8.10±0.17 eV was obtained from precision gamma-ray spectroscopy.[22]
Finally, precise measurements were achieved in 2023 by unambiguous detection of the emitted photons (8.338(24) eV)[51][52] and in April 2024 by two reports of excitation with a tunable laser at 8.355733(10) eV[53] and 8.35574(3) eV.[3][4][54][55] The light frequency is now known with sufficient accuracy to enable future construction of a prototype clock,[56][57][58] and determine the transition's exact frequency and its stability.
Precision frequency measurements began immediately, with Jun Ye's laboratory at JILA making a direct comparison to a 87
Sr
optical atomic clock. Published in September 2024, the frequency was measured as 2020407384335±2 kHz,[5][59][60][61] a relative uncertainty of 10−12. This implies a wavelength of 148.3821828827(15) nm and an energy of 8.355733554021(8) eV. The work also resolved different nuclear quadrupole sublevels and measured the ratio of the ground and excited state nuclear quadrupole moment. Improvements will surely follow.[58][62]
Applications
editWhen operational, a nuclear optical clock is expected to be applicable in various fields. In addition to the capabilities of today's atomic clocks, such as satellite-based navigation or data transfer, its high precision will allow new applications inaccessible to other atomic clocks, such as relativistic geodesy, the search for topological dark matter,[63] or the determination of time variations of fundamental constants.[64]
A nuclear clock has the potential to be particularly sensitive to possible time variations of the fine-structure constant.[65] The central idea is that the low energy is due to a fortuitous cancellation between strong nuclear and electromagnetic effects within the nucleus which are individually much stronger. Any variation the fine-structure constant would affect the electromagnetic half of this balance, resulting in a proportionally very large change in the total transition energy.[24][62] A change of even one part in 1018 could be detected by comparison with a conventional atomic clock (whose frequency would also be altered, but not nearly as much), so this measurement would be extraordinarily sensitive to any potential variation of the constant. Recent measurements and analysis are consistent with enhancement factors on the order of 104.[34][66][67][68]
References
edit- ^ a b c d e f Peik, Ekkehard; Tamm, Christian (15 January 2003). "Nuclear laser spectroscopy of the 3.5 eV transition in 229Th" (PDF). Europhysics Letters. 61 (2): 181–186. Bibcode:2003EL.....61..181P. doi:10.1209/epl/i2003-00210-x. S2CID 250818523. Archived (PDF) from the original on 2024-04-11. Retrieved 2019-03-17.
- ^ a b c d Campbell, Corey; et al. (23 March 2012). "Single-ion nuclear clock for metrology at the 19th decimal place" (PDF). Physical Review Letters. 108 (12) 120802. arXiv:1110.2490. Bibcode:2012PhRvL.108l0802C. doi:10.1103/PhysRevLett.108.120802. PMID 22540568. S2CID 40863227.
- ^ a b Thirolf, Peter (April 29, 2024). "Shedding Light on the Thorium-229 Nuclear Clock Isomer". Physics. 17 71. Bibcode:2024PhyOJ..17...71T. doi:10.1103/Physics.17.71.
- ^ a b c d e Tiedau, J.; Okhapkin, M. V.; Zhang, K.; Thielking, J.; Zitzer, G.; Peik, E.; et al. (29 April 2024). "Laser Excitation of the Th-229 Nucleus" (PDF). Physical Review Letters. 132 (18) 182501. Bibcode:2024PhRvL.132r2501T. doi:10.1103/PhysRevLett.132.182501. PMID 38759160.
The nuclear resonance for the Th4+ ions in Th:CaF2 is measured at the wavelength 148.3821(5) nm, frequency 2020.409(7) THz, and the fluorescence lifetime in the crystal is 630(15) s, corresponding to an isomer half-life of 1740(50) s for a nucleus isolated in vacuum.
- ^ a b Zhang, Chuankun; Ooi, Tian; Higgins, Jacob S.; Doyle, Jack F.; von der Wense, Lars; Beeks, Kjeld; Leitner, Adrian; Kazakov, Georgy; Li, Peng; Thirolf, Peter G.; Schumm, Thorsten; Ye, Jun (4 September 2024). "Frequency ratio of the 229mTh nuclear isomeric transition and the 87Sr atomic clock". Nature. 633 (8028): 63–70. arXiv:2406.18719. doi:10.1038/s41586-024-07839-6. PMID 39232152.
The transition frequency between the I = 5/2 ground state and the I = 3/2 excited state is determined as: 𝜈Th = 1/6 (𝜈a + 2𝜈b + 2𝜈c + 𝜈d) = 2020407384335(2) kHz.
- ^ Conover, Emily (5 September 2024). "A nuclear clock prototype hints at ultraprecise timekeeping". Science News. Also appears in the 5 October print edition (p. 7) titled "Nuclear clock prototype makes debut".
- ^ a b c d e von der Wense, Lars; Seiferle, Benedict (2 November 2020). "The 229Th isomer: prospects for a nuclear optical clock". Eur. Phys. J. A. 56 (11) 277. arXiv:2009.13633. Bibcode:2020EPJA...56..277V. doi:10.1140/epja/s10050-020-00263-0. S2CID 221995928.
- ^ a b c d e f Miller, Johanna L. (1 June 2024). "Slow-motion spectroscopy paves the way for a nuclear clock". Physics Today. Vol. 77, no. 6. pp. 12–14. doi:10.1063/pt.kvtv.jxde.
- ^ Ludlow, Andrew D.; Boyd, Martin M.; Ye, Jun; Peik, E.; Schmidt, P. O. (26 June 2015). "Optical atomic clocks" (PDF). Reviews of Modern Physics. 87 (2): 637–699. arXiv:1407.3493. Bibcode:2015RvMP...87..637L. doi:10.1103/RevModPhys.87.637. S2CID 119116973.
- ^ McGrew, W. F.; et al. (2018). "Atomic clock performance enabling geodesy below the centimetre level" (PDF). Nature (Letter). 564 (7734): 87–90. arXiv:1807.11282. Bibcode:2018Natur.564...87M. doi:10.1038/s41586-018-0738-2. PMID 30487601. S2CID 53803712.
- ^ Margolis, H. S.; Panfilo, G.; Petit, G.; Oates, C.; Ido, T.; Bize, S. (25 January 2024). "The CIPM list "Recommended values of standard frequencies": 2021 update". Metrologia. 61 (3) 035005. arXiv:2401.14537. Bibcode:2024Metro..61c5005M. doi:10.1088/1681-7575/ad3afc.
- ^ Huntemann, N.; Sanner, C.; Lipphardt, B.; Tamm, Chr.; Peik, E. (8 February 2016). "Single-ion atomic clock with 3×10−18 systematic uncertainty" (PDF). Phys. Rev. Lett. 116 (6) 063001. arXiv:1602.03908. Bibcode:2016PhRvL.116f3001H. doi:10.1103/PhysRevLett.116.063001. PMID 26918984. S2CID 19870627.
- ^ Nicholson, T. L.; Campbell, S. L.; Hutson, R. B.; Marti, G. E.; Bloom, B. J.; et al. (21 April 2015). "Systematic evaluation of an atomic clock at 2×10−18 total uncertainty" (PDF). Nature Communications. 6 6896. arXiv:1412.8261. doi:10.1038/ncomms7896. PMC 4411304. PMID 25898253.
- ^ Aeppli, Alexander; Kim, Kyungtae; Warfield, William; Safronova, Marianna S.; Ye, Jun (15 March 2024). "Clock with 8×10−19 Systematic Uncertainty" (PDF). Physical Review Letters. 133 (2) 023401. arXiv:2403.10664. Bibcode:2024PhRvL.133b3401A. doi:10.1103/PhysRevLett.133.023401. PMID 39073965.
- ^ Brewer, S. M.; et al. (15 July 2019). "27Al+ quantum-logic clock with systematic uncertainty below 10−18". Physical Review Letters. 123 (3) 033201. arXiv:1902.07694. doi:10.1103/PhysRevLett.123.033201. PMID 31386450. S2CID 119075546. Minor correction in Brewer, S. M.; et al. (1 August 2023). "Erratum: 27Al+ quantum-logic clock with systematic uncertainty below 10−18 [Physical Review Letters 123, 033201 (2019)]". Physical Review Letters. 131 059901.
- ^ a b Seiferle, Benedict; von der Wense, Lars; Thirolf, Peter G. (26 January 2017). "Lifetime measurement of the 229Th nuclear isomer". Physical Review Letters. 118 (4) 042501. arXiv:1801.05205. Bibcode:2017PhRvL.118d2501S. doi:10.1103/PhysRevLett.118.042501. PMID 28186791. S2CID 37518294.
- ^ S.V. Pineda; et al. (2025). "Radiative Decay of the 229mTh Nuclear Clock Isomer in Different Host Materials". Physical Review Research. 7. arXiv:2408.12309.
- ^ a b Kazakov, G. A.; et al. (2012). "Performance of a 229Thorium solid-state nuclear clock". New Journal of Physics. 14 (8) 083019. arXiv:1204.3268. Bibcode:2012NJPh...14h3019K. doi:10.1088/1367-2630/14/8/083019. S2CID 118341064.
- ^ a b Rellergert, W. G.; et al. (21 May 2010). "Constraining the evolution of the fundamental constants with a solid-state optical frequency reference based on the 229Th nucleus" (PDF). Physical Review Letters. 104 (20) 200802. doi:10.1103/PhysRevLett.104.200802. PMID 20867019.
- ^ a b von der Wense, L.; Zhang, C. (7 July 2020). "Concepts for direct frequency-comb spectroscopy of 229mTh and an internal-conversion-based solid-state nuclear clock". Eur. Phys. J. D. 74 (7) 146. arXiv:1905.08060. Bibcode:2020EPJD...74..146V. doi:10.1140/epjd/e2020-100582-5. S2CID 209322360.
- ^ a b B. Seiferle; et al. (2019). "Energy of the 229Th nuclear clock transition". Nature. 573 (7773): 243–246. arXiv:1905.06308. Bibcode:2019Natur.573..243S. doi:10.1038/s41586-019-1533-4. PMID 31511684. S2CID 155090121.
- ^ a b T. Sikorsky; et al. (2020). "Measurement of the 229Th isomer energy with a magnetic micro-calorimeter". Phys. Rev. Lett. 125 (14) 142503. arXiv:2005.13340. Bibcode:2020PhRvL.125n2503S. doi:10.1103/PhysRevLett.125.142503. PMID 33064540. S2CID 218900580.
- ^ Ponce, F.; et al. (2018). "Accurate measurement of the first excited nuclear state in 235U". Phys. Rev. C. 97 (5) 054310. Bibcode:2018PhRvC..97e4310P. doi:10.1103/PhysRevC.97.054310.
- ^ a b Thirolf, Peter; Seiferle, Benedict; von der Wense, Lars (2022-09-05). "From atomic to nuclear clocks". CERN Courier. Retrieved 2024-04-30.
- ^ Peik, E.; Schumm, T.; Safronova, M. S.; Pálffy, A.; Weitenberg, J.; Thirolf, P. G. (15 April 2021). "Nuclear clocks for testing fundamental physics". Quantum Science and Technology. 6 (3) 034002. arXiv:2012.09304. Bibcode:2021QS&T....6c4002P. doi:10.1088/2058-9565/abe9c2.
Due to the extremely small transition strength in 235mU (radiative lifetime ∼ 1022 s), only 229mTh qualifies for a direct laser excitation and thus for the development of a nuclear clock.
- ^ Tkalya, Eugene V.; et al. (2015). "Radiative lifetime and energy of the low-energy isomeric level in 229Th". Phys. Rev. C. 92 (5) 054324. arXiv:1509.09101. Bibcode:2015PhRvC..92e4324T. doi:10.1103/PhysRevC.92.054324. S2CID 118374372.
- ^ Minkov, N.; Pálffy, A. (2017). "Reduced transition probabilities for the gamma decay of the 7.8 eV isomer in 229mTh". Physical Review Letters. 118 (21) 212501. arXiv:1704.07919. Bibcode:2017PhRvL.118u2501M. doi:10.1103/PhysRevLett.118.212501. PMID 28598657. S2CID 40694257.
- ^ Varga, Z.; Nicholl, A.; Mayer, K. (2014). "Determination of the 229Th half-life". Phys. Rev. C. 89 064310. doi:10.1103/PhysRevC.89.064310.
- ^ Tkalya, Eugene V.; et al. (March 1996). "Processes of the nuclear isomer 229mTh(3/2+, 3.5±1.0 eV) Resonant excitation by optical photons". Physica Scripta. 53 (3): 296–299. Bibcode:1996PhyS...53..296T. doi:10.1088/0031-8949/53/3/003. S2CID 250744766.
- ^ Tkayla, Eugene V. (3 November 2010). "Proposal for a nuclear gamma-ray laser of optical range". Physical Review Letters. 106 (16) 162501. arXiv:1011.0858. doi:10.1103/PhysRevLett.106.162501. PMID 21599361.
A possibility of the amplification of the 7.6 eV γ-radiation by the stimulated γ-emission of the ensemble of the 229mTh isomeric nuclei in a host dielectric crystal is proved theoretically.
- ^ Zyga, Lisa (May 2, 2011). "Proposed gamma-ray laser could emit 'nuclear light'". Phys.org.
- ^ Harris, David (April 22, 2011). "Laser from Atomic Nuclei". Physical Review Focus. Vol. 27.
A laser that emits visible light using atomic nuclei, rather than electrons, could be made from a thorium alloy. It could be a first step toward a gamma-ray laser.
- ^ Campbell, C. J.; Radnaev, A. G.; Kuzmich, A. (2 June 2011). "Wigner crystals of 229Th for optical excitation of the nuclear isomer". Physical Review Letters. 106 (22) 223001. arXiv:1110.2339. doi:10.1103/PhysRevLett.106.223001. PMID 21702597. S2CID 20801170.
- ^ a b c Thielking, Johannes; et al. (2018). "Laser spectroscopic characterization of the nuclear-clock isomer 229mTh". Nature. 556 (7701): 321–325. arXiv:1709.05325. Bibcode:2018Natur.556..321T. doi:10.1038/s41586-018-0011-8. PMID 29670266. S2CID 4990345.
- ^ Kroger, L. A.; Reich, C. W. (1976). "Features of the low energy level scheme of 229Th as observed in the α decay of 233U". Nucl. Phys. A. 259 (1): 29–60. Bibcode:1976NuPhA.259...29K. doi:10.1016/0375-9474(76)90494-2.
- ^ a b Reich, C. W.; Helmer, R. G. (15 January 1990). "Energy separation of the doublet of intrinsic states at the ground state of 229Th". Phys. Rev. Lett. 64 (3). American Physical Society: 271–273. Bibcode:1990PhRvL..64..271R. doi:10.1103/PhysRevLett.64.271. PMID 10041937.
It has been known for some time that the intrinsic state labeled by the asymptotic quantum numbers 3/2+[631] lies quite close (< 0.1 keV) to the 5/2+[633] ground state of 229Th. Using the energies of selected γ rays emitted following the α decay of 233U, we have obtained a value of 1±4 eV for the energy separation of these two intrinsic states.
- ^ a b von der Wense, Lars (2016). On the direct detection of 229mTh (PDF) (PhD thesis). Ludwig Maximilian University of Munich. ISBN 978-3-319-70461-6.
- ^ Helmer, R. G.; Reich, C. W. (1994). "An Excited State of 229Th at 3.5 eV". Phys. Rev. C. 49 (4): 1845–1858. Bibcode:1994PhRvC..49.1845H. doi:10.1103/PhysRevC.49.1845. PMID 9969412.
- ^ Fused Quartz and Fused Silica for Optical Applications: Data and Properties (PDF) (Catalogue). Heraeus. February 2023. p. 6. 8 eV corresponds to 155 nm wavelength, where even the highest-purity fused silica becomes opaque.
- ^ B.R. Beck; et al. (2007). "Energy splitting in the ground state doublet in the nucleus 229Th". Phys. Rev. Lett. 98 (14) 142501. Bibcode:2007PhRvL..98n2501B. doi:10.1103/PhysRevLett.98.142501. PMID 17501268.
- ^ B.R. Beck; et al. (15–19 June 2009). Improved value for the energy splitting of the ground-state doublet in the nucleus 229mTh (PDF). 12th Int. Conf. on Nuclear Reaction Mechanisms. Varenna, Italy. LLNL-PROC-415170. Archived (PDF) from the original on 2017-01-27. Retrieved 2024-09-02.
- ^ Jeet, Justin; et al. (23 June 2015). "Results of a Direct Search Using Synchrotron Radiation for the Low-Energy 229Th Nuclear Isomeric Transition". Physical Review Letters. 114 (25) 253001. arXiv:1502.02189. Bibcode:2015PhRvL.114y3001J. doi:10.1103/physrevlett.114.253001. PMID 26197124. S2CID 1322253.
- ^ Yamaguchi, A.; et al. (28 May 2015). "Experimental search for the low-energy nuclear transition in 229Th with undulator radiation". New Journal of Physics. 17 (5) 053053. Bibcode:2015NJPh...17e3053Y. doi:10.1088/1367-2630/17/5/053053. ISSN 1367-2630.
- ^ Stellmer, Simon; Kazakov, Georgy; Schreitl, Matthias; Kaser, Hendrik; Kolbe, Michael; Schumm, Thorsten (18 June 2018). "Attempt to optically excite the nuclear isomer in 229Th". Phys. Rev. A. 97 (6) 062506. arXiv:1803.09294. Bibcode:2018PhRvA..97f2506S. doi:10.1103/PhysRevA.97.062506. S2CID 4946329.
- ^ Zhao, X.; et al. (2012). "Observation of the Deexcitation of the 229mTh Nuclear Isomer". Physical Review Letters. 109 (16) 160801. Bibcode:2012PhRvL.109p0801Z. doi:10.1103/PhysRevLett.109.160801. ISSN 0031-9007. PMID 23215066.
- ^ Borisyuk, P. V.; et al. (2018). "Excitation of 229Th nuclei in laser plasma: the energy and half-life of the low-lying isomeric state". arXiv:1804.00299 [nucl-th].
- ^ Peik, E.; Zimmermann, K. (2013). "Comment on "Observation of the Deexcitation of the 229mTh Nuclear Isomer"". Physical Review Letters. 111 (1) 018901. Bibcode:2013PhRvL.111a8901P. doi:10.1103/PhysRevLett.111.018901. PMID 23863029.
- ^ von der Wense, Lars; et al. (5 May 2016). "Direct detection of the 229Th nuclear clock transition". Nature. 533 (7601): 47–51. arXiv:1710.11398. Bibcode:2016Natur.533...47V. doi:10.1038/nature17669. PMID 27147026. S2CID 205248786.
- ^ Masuda, Takahiko; Yoshimi, Akihiro; Fujieda, Akira; Fujimoto, Hiroyuki; Haba, Hiromitsu; Hara, Hideaki; et al. (2019). "X-ray pumping of the 229Th nuclear clock isomer". Nature. 573 (7773): 238–242. arXiv:1902.04823. Bibcode:2019Natur.573..238M. doi:10.1038/s41586-019-1542-3. PMID 31511686. S2CID 119083861.
- ^ A. Yamaguchi; et al. (2019). "Energy of the 229Th nuclear clock isomer determined by absolute gamma-ray energy difference". Phys. Rev. Lett. 123 (22) 222501. arXiv:1912.05395. Bibcode:2019PhRvL.123v2501Y. doi:10.1103/PhysRevLett.123.222501. PMID 31868403. S2CID 209202193.
- ^ Conover, Emily (June 1, 2023). "Measurements of a key radioactive decay nudge a nuclear clock closer to reality". ScienceNews.
- ^ Kraemer, Sandro; et al. (May 25, 2023). "Observation of the radiative decay of the 229Th nuclear clock isomer". Nature. 617 (7962): 706–710. arXiv:2209.10276. Bibcode:2023Natur.617..706K. doi:10.1038/s41586-023-05894-z. PMID 37225880.
photons of 8.338(24) eV are measured, in agreement with recent measurements and the uncertainty is decreased by a factor of seven. The half-life of 229mTh embedded in MgF2 is determined to be 670(102) s
- ^ Elwell, R.; Schneider, Christian; Jeet, Justin; Terhune, J. E. S.; Morgan, H. W. T.; Alexandrova, A. N.; Tran Tan, Hoang Bao; Derevianko, Andrei; Hudson, Eric R. (2 July 2024). "Laser excitation of the 229Th nuclear isomeric transition in a solid-state host". Physical Review Letters. 133 (1) 013201. arXiv:2404.12311. doi:10.1103/PhysRevLett.133.013201. PMID 39042795.
a narrow, laser-linewidth-limited spectral feature at 148.38219(4)stat(20)sys nm (2020407.3(5)stat(30)sys GHz) that decays with a lifetime of 568(13)stat(20)sys s. This feature is assigned to the excitation of the 229Th nuclear isomeric state, whose energy is found to be 8.355733(2)stat(10)sys eV in 229Th:LiSrAlF6.
- ^ Klapetz, Patrick (2024-04-29). "Thorium-229: Erstmals Atomkern mit Laser angeregt" [Thorium-229: First atomic nucleus excited by laser]. Golem.de: IT-News für Profis (in German). Archived from the original on 2024-04-30. Retrieved 2024-04-30.
- ^ "We did it !" (Press release). Thoriumclock.eu project. 29 April 2024.
We are overly excited to announce: Our teams from TU Wien and PTB Braunschweig successfully managed the laser excitation of the Th-229 Nucleus.
- ^ Thirolf, Peter G.; et al. (March 2020). "'Phase Transition' in the 'Thorium-Isomer Story'". Acta Physica Polonica B. 51 (3): 561–570. arXiv:2108.13388. Bibcode:2020AcPPB..51..561T. doi:10.5506/APhysPolB.51.561. Originally presented as Characterization of the elusive 229mTh isomer – milestones towards a nuclear clock.
- ^ Gibney, Elizabeth (8 May 2024). "Best ever clocks: breakthrough paves way for ultra-precise 'nuclear' timekeepers". Nature. 629 (8012): 514–515. Bibcode:2024Natur.629..514G. doi:10.1038/d41586-024-01353-5. PMID 38719967.
To turn the system into an actual clock, physicists will need to markedly reduce the resolution of the laser, so that it stimulates the nucleus at almost exactly the right frequency to be read off reliably, says Peik. Building such a laser 'remains a big challenge, but there are little doubts that it will be achievable in the near future', adds Kocharovskaya.
- ^ a b Conover, Emily (4 April 2024). "Physicists take a major step toward making a nuclear clock". ScienceNews.
There's still much more work to be done to build a nuclear clock. And even once scientists have built them, Ye says, 'it will take years, if not decades, of work to catch up with atomic clocks.' But 'just being able to see the transition opens the door.'
- ^ Conover, Emily (4 September 2024). "A nuclear clock prototype hints at ultraprecise timekeeping". ScienceNews.
- ^ "Major leap for nuclear clock paves way for ultraprecise timekeeping" (Press release). NIST. 4 September 2024.
- ^ "The first ultra precised nuclear clock is built!" (Press release). Thoriumclock.eu project. 4 September 2024.
While this laboratory demonstration is not a fully developed nuclear clock, it contains all the core technology for one.
- ^ a b Howlett, Joseph (4 September 2024). "The First Nuclear Clock Will Test if Fundamental Constants Change". Quanta Magazine.
- ^ Derevianko, A.; Pospelov, M. (December 2014). "Hunting for topological dark matter with atomic clocks". Nature Physics. 10 (12): 933–936. arXiv:1311.1244. Bibcode:2014NatPh..10..933D. doi:10.1038/nphys3137. S2CID 53630878.
- ^ Thirolf, P. G.; Seiferle, B.; von der Wense, L. (2019). "Improving Our Knowledge on the 229mThorium Isomer: Toward a Test Bench for Time Variations of Fundamental Constants". Annalen der Physik. 531 (5) 1800381. Bibcode:2019AnP...53100381T. doi:10.1002/andp.201800381.
- ^ Flambaum, V. V. (September 2006). "Enhanced Effect of Temporal Variation of the Fine Structure Constant and the Strong Interaction in 229mTh". Physical Review Letters. 97 (9) 092502. arXiv:physics/0601034. doi:10.1103/PhysRevLett.97.092502. PMID 17026357. S2CID 4109230.
- ^ Fadeev, Pavel; Berengut, Julian C.; Flambaum, Victor V. (November 2020). "Sensitivity of 229Th nuclear clock transition to variation of the fine-structure constant". Physical Review A. 102 (5) 052833. arXiv:2007.00408. Bibcode:2020PhRvA.102e2833F. doi:10.1103/PhysRevA.102.052833.
The enhancement factor for α variation is K = −(0.82±0.25)×104.
- ^ Caputo, Andrea; Gazit, Doron; Hammer, Hans-Werner; Kopp, Joachim; Paz, Gil; Perez, Gilad; Springmann, Konstantin (22 July 2024). "On the sensitivity of nuclear clocks to new physics". arXiv:2407.17526 [hep-ph].
We find that the nuclear clock's sensitivity to variations in the effective fine structure constant is enhanced by a factor of order 104.
- ^ Beeks, Kjeld; Kazakov, Georgy A.; Schaden, Fabian; Morawetz, Ira; de Col, Luca Toscani; Riebner, Thomas; Bartokos, Michael; Sikorsky, Tomas; Schumm, Thorsten; Zhang, Chuankun; Ooi, Tian; Higgins, Jacob S.; Doyle, Jack F.; Ye, Jun; Safronova, Marianna S. (24 July 2024). "Fine-structure constant sensitivity of the Th-229 nuclear clock transition". arXiv:2407.17300 [nucl-th].
this allows to quantify the sensitivity of α to K=5900(2300)