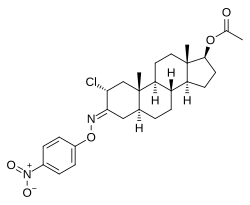
Nisterime acetate (USAN) (developmental code name ORF-9326), also known as 2α-chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate or as 2α-chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was developed as a postcoital contraceptive but was never marketed.[2][3][1] It is an androgen ester – specifically, the C17α acetate ester of nisterime.[2] Unlike antiprogestogens like mifepristone, nisterime acetate does not prevent implantation and instead induces embryo resorption as well as interrupts the post-implantation stage of pregnancy.[4]
 | |
| Clinical data | |
|---|---|
| Other names | 2α-Chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate; 2α-Chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate; 2α-Chloro-3-(p-nitrophenoxy)imino-5α-androstan-17β-ol 17β-acetate |
| Routes of administration | Oral[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H35ClN2O5 |
| Molar mass | 503.04 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nisterime acetate is described as an androgen by some sources.[2][5] However, it has also been reported that the drug lacks hormonal activity in bioassays, including androgenic, estrogenic, or progestogenic activity (as well as antagonistic activity).[1][6] This finding has been described as puzzling in light of the potent abortifacient activity of the drug in animals and it has been said that its mechanism of action remains unknown.[6]
See also
editReferences
edit- ^ a b c Hahn DW, Allen GO, Greenslade FC (1977). "The Post-Implantive Contragestational Activity of 17β-acetoxy-2α-chloro-3-(p-nitrophenoxy) imino-5α androstane (ORF 9326) in the Rat". Federation Proceedings. 36 (3): 342. S2CID 74670821.
- ^ a b c Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 876–. ISBN 978-1-4757-2085-3.
- ^ Patrick JE, Weintraub HS, McGuire JL (September 1978). "Disposition of ORF 9326, a novel contragestational steroid, in animals". Steroids. 32 (2): 147–156. doi:10.1016/0039-128x(78)90001-6. PMID 102053. S2CID 106098.
- ^ Hahn DW, McGuire JL (6 December 2012). "The Use of Pharmacological Agents to Study Implantation". In Glasser SR, Bullock DW (eds.). Cellular and Molecular Aspects of Implantation. Springer Science & Business Media. pp. 479–. ISBN 978-1-4613-3180-3.
- ^ "Nisterime acetate". KEGG DRUG.
- ^ a b Zatuchni GI, Labbok MH, Sciarra JJ, eds. (1980). Research frontiers in fertility regulation. Harper & Row. p. 368. ISBN 978-0-06-142902-6.
ORF 9326 (17β-acetoxy-2α-chloro-3(p-nitrophenoxy) imino-5-androstane) is a derivative of dihydrotestosterone (Fig. 33-1). This compound was found to interrupt pregnancy in a number of species, but the fact that it has no hormonal activity is puzzling and its mechanism of action is still unknown.