Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation.[1]

| |
| |
| Names | |
|---|---|
| IUPAC names
Niobium(V) chloride
Niobium pentachloride | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.042 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| NbCl5 | |
| Molar mass | 270.17 g/mol |
| Appearance | yellow monoclinic crystals deliquescent |
| Density | 2.75 g/cm3 |
| Melting point | 204.7 °C (400.5 °F; 477.8 K) |
| Boiling point | 248.2 °C (478.8 °F; 521.3 K) |
| decomposes | |
| Solubility | HCl, chloroform, CCl4 |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
214.05 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
-797.47 kJ/mol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Niobium(V) fluoride Niobium(V) bromide Niobium(V) iodide |
Other cations
|
Vanadium(IV) chloride Tantalum(V) chloride |
Related niobium chlorides
|
Niobium(III) chloride Niobium(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
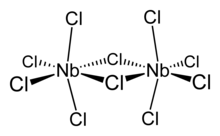
Structure and properties
editNiobium(V) chloride forms chloro-bridged dimers in the solid state (see figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction.[2] NbBr5, NbI5, TaCl5 TaBr5 and TaI5 are isostructural with NbCl5.
Preparation
editIndustrially, niobium pentachloride is obtained by direct chlorination of niobium metal at 300 to 350 °C:[3]
- 2 Nb + 5 Cl2 → 2 NbCl5
In the laboratory, niobium pentachloride is often prepared from Nb2O5, the main challenge being incomplete reaction to give NbOCl3. The conversion can be effected with thionyl chloride:[4] It also can be prepared by chlorination of niobium pentoxide in the presence of carbon at 300 °C.
Uses
editNiobium(V) chloride is the main precursor to the alkoxides of niobium, which find uses in sol-gel processing. It is also the precursor to many other Nb-containing reagents, including most organoniobium compounds.
In organic synthesis, NbCl5 is a very specialized Lewis acid in activating alkenes for the carbonyl-ene reaction and the Diels-Alder reaction. Niobium chloride can also generate N-acyliminium compounds from certain pyrrolidines which are substrates for nucleophiles such as allyltrimethylsilane, indole, or the silyl enol ether of benzophenone.[5]
References
edit- ^ Cotton, F. Albert; Wilkinson, Geoffrey (1980), Advanced Inorganic Chemistry (4th ed.), New York: Wiley, ISBN 0-471-02775-8
- ^ Cotton, F.A., P. A. Kibala, M. Matusz and R. B. W. Sandor (1991). "Structure of the Second Polymorph of Niobium Pentachloride". Acta Crystallogr. C. 47 (11): 2435–2437. doi:10.1107/S0108270191000239.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Joachim Eckert; Hermann C. Starck (2005). "Niobium and Niobium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_251. ISBN 3-527-30673-0.
- ^ Brown, D. (1957). "Niobium(V) Chloride and Hexachloroniobates(V)". Inorganic Syntheses. Vol. 9. pp. 88–92. doi:10.1002/9780470132401.ch24. ISBN 978-0-470-13240-1.
- ^ Andrade, C. K. Z.; Rocha, R. O.; Russowsky, D. & Godoy, M. N. (2005). "Studies on the Niobium Pentachloride-Mediated Nucleophilic Additions to an Enantiopure Cyclic N-acyliminium Ion Derived from (S)-malic acid". J. Braz. Chem. Soc. 16 (3b): 535–539. doi:10.1590/S0103-50532005000400007. hdl:10183/24558.