Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.

| |
| |
| Names | |
|---|---|
| IUPAC name
Nickel(II) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.053 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NiF2 | |
| Molar mass | 96.6902 g/mol |
| Appearance | Yellowish to green tetragonal crystals |
| Density | 4.72 g/cm3 |
| Melting point | 1,474 °C (2,685 °F; 1,747 K)[1] |
| 4 g/100 mL | |
| Solubility | insoluble in alcohol, ether |
| +2410.0·10−6 cm3/mol | |
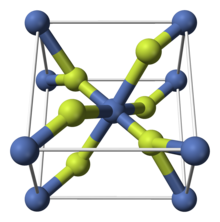
| Structure | |
| Rutile | |
| Nickel: Octahedral Oxygen: Trigonal planar | |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Nickel(II) chloride Nickel(II) bromide Nickel(II) iodide |
Other cations
|
Cobalt(II) fluoride Copper(II) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nickel(II) fluoride is also produced when nickel metal is exposed to fluorine. In fact, NiF2 comprises the passivating surface that forms on nickel alloys (e.g. monel) in the presence of hydrogen fluoride or elemental fluorine. For this reason, nickel and its alloys are suitable materials for storage and transport these fluorine and related fluorinating agents. NiF2 is also used as a catalyst for the synthesis of chlorine pentafluoride.
Preparation and structure
editNiF2 is prepared by treatment of anhydrous nickel(II) chloride with fluorine at 350 °C:[2]
- NiCl2 + F2 → NiF2 + Cl2
The corresponding reaction of cobalt(II) chloride results in oxidation of the cobalt, whereas nickel remains in the +2 oxidation state after fluorination because its +3 oxidation state is less stable. Chloride is more easily oxidized than nickel(II). This is a typical halogen displacement reaction, where a halogen plus a less active halide makes the less active halogen and the more active halide.
Like some other metal difluorides, NiF2 crystallizes in the rutile structure, which features octahedral Ni centers and planar fluorides.[3] At low temperatures, its magnetic structure is antiferromagnetic.[4]
Reactions
editA melt of NiF2 and KF reacts to give successively potassium trifluoronickelate and potassium tetrafluoronickelate:[5]
- NiF2 + KF → K[NiF3]
- K[NiF3] + KF → K2[NiF4]
The structure of this material is closely related to some superconducting oxide materials.[6]
Nickel(II) fluoride reacts with strong bases to give nickel(II) hydroxide:
- NiF2 + 2 NaOH → Ni(OH)2 + 2 NaF
References
edit- ^ Planning. Validations stanford.edu [dead link]
- ^ Priest, H. F. (1950). "Anhydrous Metal Fluorides". Inorganic Syntheses. Vol. 3. pp. 171–183. doi:10.1002/9780470132340.ch47. ISBN 9780470132340.
- ^ Stout, J. W.; Reed, Stanley A. (1954). "The Crystal Structure of MnF2, FeF2, CoF2, NiF2 and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ^ Strempfer, J; Ruett, U; Bayrakci, S.P.; Brueckel, Th.; Jauch, W (2004). "Magnetic properties of transition metal fluorides". Phys. Rev. B. 69: 014417. doi:10.1103/PhysRevB.69.014417.
- ^ Plevey, R. G.; Rendell, R. W.; Steward, M. P. (1974-01-01). "Fluorination with complex metal fluorides Part III. The fluorination of benzene over potassium hexafluoronickelate(IV)". Journal of Fluorine Chemistry. 3 (3–4): 267–273. Bibcode:1974JFluC...3..267P. doi:10.1016/S0022-1139(00)82626-3. ISSN 0022-1139.
- ^ Balz, D. (1953). "Über die Struktur des K2NiF4". Naturwissenschaften. 40 (8): 241. Bibcode:1953NW.....40..241B. doi:10.1007/BF00591545. S2CID 32692990.