Nabiximols (USAN)[2] sold under the brand name Sativex, is a specific Cannabis extract that was approved in 2010 as a botanical drug in the United Kingdom. Nabiximols is sold as a mouth spray intended to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis; it was developed by the UK company GW Pharmaceuticals.[3][4] In 2019, it was proposed that following application of the spray, nabiximols is washed away from the oral mucosa by the saliva flow and ingested into the stomach, with subsequent absorption from the gastro-intestinal tract.[5][6] Nabiximols is a combination drug standardized in composition, formulation, and dose. Its principal active components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). Each spray delivers a dose of 2.7 mg THC and 2.5 mg CBD.
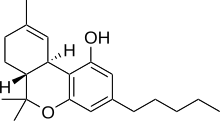 | |
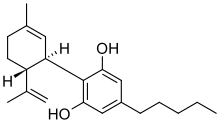 Chemical structures of tetrahydrocannabinol (top) and cannabidiol (bottom) | |
| Combination of | |
|---|---|
| Tetrahydrocannabinol | Cannabinoid |
| Cannabidiol | Cannabinoid |
| Clinical data | |
| Trade names | Sativex |
| Routes of administration | Oromucosal spray |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| | |

In 2003, GW Pharmaceuticals partnered with Bayer to market the drug under the brand name Sativex. In 2011, GW licensed the rights to commercialise nabiximols to Novartis for Asia (excluding China and Japan), Africa and the Middle East (excluding Israel).[7]
Availability
editIn June 2010, the Medicines and Healthcare products Regulatory Agency of the United Kingdom licensed nabiximols as a prescription-only medicine for the treatment of spasticity due to multiple sclerosis. This regulatory authorization represents the world's first full regulatory approval for the medicine. The spray is being marketed in the UK by Bayer Schering Pharma. Many people with MS cannot receive nabiximols due to local National Health Service (NHS) resistance to its funding;[8][9] but, in August 2014, the NHS in Wales agreed to fund Sativex for people with multiple sclerosis.[10]
Nabiximols was also approved in Spain for MS spasticity in the second half of 2010, and was launched in that country in March 2011. It was approved in the Czech Republic in April 2011, in Germany in May 2011, in Denmark in June 2011, and in Sweden in January 2012 to people with MS who have not responded adequately to other medication for spasticity.[11] It has also been recommended for approval in Italy and Austria with formal approvals expected in these countries during 2011. In Spain and other European markets (excluding the UK), nabiximols will be marketed by Almirall.
In Canada, nabiximols has been approved by Health Canada for the treatment of MS spasticity. It has also received a licence with conditions (NOC/c) for two additional uses: as adjunctive treatment for the symptomatic relief of neuropathic pain in multiple sclerosis,[12] and also for pain due to cancer.[13][14]
Nabiximols is available in a number of countries as an unlicensed medicine, which enables doctors to prescribe the product to people who they consider may benefit. The product has been exported from the UK to a total of 28 countries to date.
In February 2007, GW and Otsuka Pharmaceutical announced an exclusive agreement for Otsuka to develop and market the drug in the United States. The first large scale US Phase IIb trial, Spray Trial, for people with cancer reported positive results in March 2010. GW and Otsuka have now commenced the Phase III development of nabiximols in cancer pain.
In December 2012, Sativex was approved in Poland.[15]
In 2013, France legalized the use of cannabinoids in medicine, Sativex is the first one to be sold under prescription.[16] Nevertheless, as of June 2016 this drug had still not actually been sold in pharmacies there.[17]
Effectiveness
editOf the two preliminary Phase III studies investigating the treatment of people with MS, one showed a reduction of spasticity of 1.2 points on the 0–10 points rating scale (versus 0.6 points under placebo), the other showed a reduction of 1.0 versus 0.8 points. Only the first study reached statistical significance. The Phase III approval study consisted of a run-in phase where the response of individuals to the drug was determined. The responders (42% of subjects) showed a significant effect in the second, placebo controlled, phase of the trial.[18] A 2009 meta-analysis of six studies found large variations of effectiveness, with a – statistically non-significant – trend towards a reduction of spasticity.[19] A systematic review in 2014 by the American Academy of Neurology found that nabiximols was 'probably effective' for spasticity, pain, and urinary dysfunction, but wasn't supported for tremor.[20] A 2021 study, however, showed “clinically relevant symptomatic results”[21]
Nabiximols has also been studied for cancer pain resistant to opioids. While adjuvant use of nabiximols was safe in 3 trials for cancer pain,[22][23][24] data regarding efficacy were mixed, and the drug failed to meet its primary endpoint for this purpose in its first Phase III trial.[25]
Side effects
editIn early clinical trials, nabiximols has generally been well tolerated.[26][27][28] The most common adverse effects in Phase III trials were dizziness (25%), drowsiness (8%) and disorientation (4%); 12% of subjects stopped taking the drug because of the side effects. No investigations regarding the potential for dependence are available, but such a potential is unlikely considering the pharmacological properties of the two components.[18] A systematic review has shown no evidence is available about the negative effect of Nabiximols on cognition ability [29]
Licensing
editGW Pharmaceuticals were issued a license to cultivate cannabis for the manufacturing of Sativex in the United Kingdom (UK), granting them the sole legal right to research in aerosolized cannabis derived therapeutics, which became commercially viable in April 2013, when the UK Government scheduled the Sativex formulation to part IV of the UK Drugs Act.[30]
See also
editReferences
edit- ^ "DDrare: Database of Drug Development for Rare Diseases". ホーム | DDrare (in Japanese). 2016-12-28. Retrieved 2024-03-23.
- ^ "Statement on a non-proprietary name" (PDF). United States Adopted Names Council.
- ^ "Nabiximols". UK Medicines Online.[permanent dead link] Page accessed Feb 3, 2016
- ^ "Sativex (nabiximols) - factsheet". Multiple Sclerosis Trust. October 2014.
- ^ Itin C, Barasch D, Domb AJ, Hoffman A (May 2020). "Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol". International Journal of Pharmaceutics. 581: 119276. doi:10.1016/j.ijpharm.2020.119276. PMID 32243971. S2CID 214785913.
- ^ Itin C, Domb AJ, Hoffman A (October 2019). "A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states". Expert Opinion on Drug Delivery. 16 (10): 1031–1035. doi:10.1080/17425247.2019.1653852. PMID 31393180. S2CID 199505274.
- ^ "GW signs Sativex cannabis-based drug deal with Novartis". The Telegraph. 11 April 2011. Retrieved 12 July 2012.
- ^ Ryan S (4 June 2011). "Sussex MS sufferers call for drug funding". Argus (Sussex, UK). Retrieved 8 June 2011.
- ^ "Sativex rejected by healthcare provider". Lincolnshire. 20 June 2011. Archived from the original on 22 June 2011. Retrieved 20 June 2011.
- ^ "Wales NHS to offer MS cannabis drug Sativex". 15 August 2014. Retrieved 18 August 2014.
- ^ "Sativex (nabiximols)". Swedish Medical Products Agency. Archived from the original on 2014-01-01.
- ^ "Multiple Sclerosis". GW Pharmaceuticals\. Archived from the original on 2011-08-11. Retrieved 24 July 2011.
- ^ "Cancer Pain". GW Pharmaceuticals. Archived from the original on 2011-09-27. Retrieved 24 July 2011.
- ^ "Sativex - Investigational Cannabis-Based Treatment for Pain and Multiple Sclerosis Drug Development Technology". www.drugdevelopment-technology.com. Retrieved 2008-08-08.
- ^ Olszewska D, Kidawa M. "Sativex - lek z marihuany". Krajowe Biuro Do Spraw Przeciwdziałania Narkomanii.
- ^ "France Legalizes Marijuana-Based Drug To Treat Multiple Sclerosis". HunffingtonPost. Retrieved 4 June 2015.
- ^ "Cannabis thérapeutique : pourquoi le Sativex n'est-il toujours pas vendu en France ?". Sciences et Avenir. Archived from the original on 2016-05-10. Retrieved 6 June 2016.
- ^ a b Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2011/2012 (in German)
- ^ Lakhan SE, Rowland M (December 2009). "Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review". BMC Neurology. 9: 59. doi:10.1186/1471-2377-9-59. PMC 2793241. PMID 19961570.
- ^ Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (April 2014). "Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology". Neurology. 82 (17): 1556–1563. doi:10.1212/WNL.0000000000000363. PMC 4011465. PMID 24778283.
- ^ D'hooghe M, Willekens B, Delvaux V, D'haeseleer M, Guillaume D, Laureys G, et al. (June 2021). "Sativex® (nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: the Belgian experience". BMC Neurology. 21 (1): 227. doi:10.1186/s12883-021-02246-0. PMC 8218396. PMID 34157999. S2CID 235496708.
- ^ Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. (May 2012). "Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial". The Journal of Pain. 13 (5): 438–449. doi:10.1016/j.jpain.2012.01.003. PMID 22483680.
- ^ Lynch ME, Cesar-Rittenberg P, Hohmann AG (January 2014). "A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain". Journal of Pain and Symptom Management. 47 (1): 166–173. doi:10.1016/j.jpainsymman.2013.02.018. PMID 23742737.
- ^ Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT (August 2013). "An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics". Journal of Pain and Symptom Management. 46 (2): 207–218. doi:10.1016/j.jpainsymman.2012.07.014. PMID 23141881.
- ^ Underwood G (9 January 2015). "Last stage failure for Otsuka and GW Cancer Pain Drug". Pharmafile. Retrieved 2016-12-07.
- ^ Wade DT, Makela P, Robson P, House H, Bateman C (August 2004). "Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients". Multiple Sclerosis. 10 (4): 434–441. doi:10.1191/1352458504ms1082oa. PMID 15327042. S2CID 1378674.
- ^ Wade DT, Makela PM, House H, Bateman C, Robson P (October 2006). "Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis". Multiple Sclerosis. 12 (5): 639–645. doi:10.1177/1352458505070618. PMID 17086911. S2CID 16175440.
- ^ Wade DT, Robson P, House H, Makela P, Aram J (February 2003). "A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms". Clinical Rehabilitation. 17 (1): 21–29. doi:10.1191/0269215503cr581oa. PMID 12617376. S2CID 1414988.
- ^ Motaghi E, Ghasemi-Pirbaluti M, Rashidi M, Alasvand M, Di Ciano P, Bozorgi H (March 2023). "The effect of tetrahydrocannabinol:cannabidiol oromucosal spray on cognition: a systematic review". European Journal of Clinical Pharmacology. 79 (3): 371–381. doi:10.1007/s00228-023-03454-y. PMID 36700997. S2CID 256273374.
- ^ "GWPharma - GW Pharmaceuticals' cannabinoid-medicine Sativex® moved to Schedule 4 of UK Drugs Act". Archived from the original on 2013-10-29. Retrieved 2013-11-27.