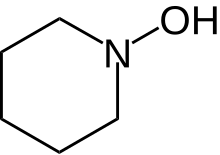
N-Hydroxypiperidine (also known as 1-piperidinol and 1-hydroxypiperidine) is the chemical compound with formula C5H11NO. It is a hydroxylated derivative of the heterocyclic compound piperidine.
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidin-1-ol | |
| Other names
1-Hydroxypiperidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 102726 | |
| ChemSpider | |
| ECHA InfoCard | 100.023.057 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H11NO | |
| Molar mass | 101.149 g·mol−1 |
| Appearance | Fine white crystals |
| Density | 1.070 g/cm3 |
| Melting point | 39.3 °C (102.7 °F; 312.4 K) |
| Boiling point | 98.5 °C (209.3 °F; 371.6 K) |
| 113 g/L | |
| log P | -0.17 |
| Vapor pressure | 0.542 Torr |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 84.9 °C (184.8 °F; 358.0 K) |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editN-Hydroxypiperidine can be prepared from the application of meta-chloroperoxybenzoic acid and methanol to the tertiary amine product of acrylonitrile and piperidine, followed by heating with acetone of the resulting tertiary N-oxide.[1]
Reactions
editN-Hydroxypiperidine is a secondary amine, which can undergo an oxidation reaction with hydrogen peroxide in methanol as the solvent. This produces a nitrone, which is heteroatomic equivalent to a ketone with a nitrogen instead of an alpha carbon. Competing elimination reactions can occur, as well.[2]
References
edit- ^ O’Neil, I. A.; Cleator, E.; Tapolczay, D. J. A convenient synthesis of secondary hydroxylamines. Tetrahedron Letters, 2001, 42, pp. 8247–8249
- ^ Zauche, Timothy H.; Espenson, James H. (1997). "Kinetics and Mechanism of the Oxidation of Secondary Hydroxylamines to Nitrones with Hydrogen Peroxide, Catalyzed by Methylrhenium Trioxide". Inorganic Chemistry. 36 (23): 5257. doi:10.1021/ic970649d.
