Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand (a pterin) that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor.

| |
| Names | |
|---|---|
| IUPAC name
[2-amino-4-oxo-6,7-bis(sulfanyl)-3,5,5~{a},8,9~{a},10-hexahydropyrano[3,2-g]pteridin-8-yl]methyl dihydrogen phosphate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| MeSH | molybdopterin |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C 10H 10N 5O 6PS 2 + R groups | |
| Molar mass | 394.33 g/mol (R=H) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Molybdopterin consists of a pyranopterin, a complex heterocycle featuring a pyran fused to a pterin ring. In addition, the pyran ring features two thiolates, which serve as ligands in molybdo- and tungstoenzymes. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. Enzymes that contain the molybdopterin cofactor include xanthine oxidase, DMSO reductase, sulfite oxidase, and nitrate reductase.
The only molybdenum-containing enzymes that do not feature molybdopterins are the nitrogenases (enzymes that fix nitrogen). These contain an iron-sulfur center of a very different type, which also contains molybdenum.[5]
Biosynthesis
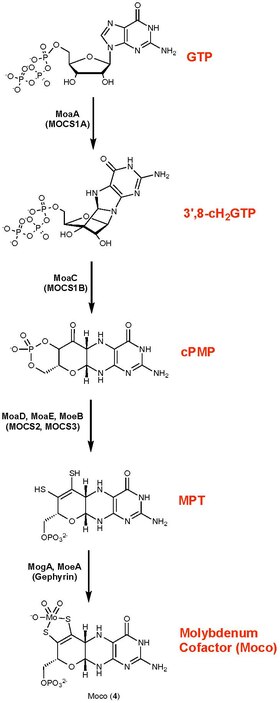
editUnlike many other cofactors, molybdenum cofactor (Moco) cannot be taken up as a nutrient. The cofactor thus requires de novo biosynthesis. Molybdenum cofactor biosynthesis occurs in four steps: (i) the radical-mediated cyclization of nucleotide, guanosine triphosphate (GTP), to (8S)‑3',8‐cyclo‑7,8‑dihydroguanosine 5'‑triphosphate (3',8‑cH2GTP), (ii) the formation of cyclic pyranopterin monophosphate (cPMP) from the 3',8‑cH2GTP, (iii) the conversion of cPMP into molybdopterin (MPT), (iv) the insertion of molybdate into MPT to form Moco.[6][7]
Two enzyme-mediated reactions convert guanosine triphosphate to the cyclic phosphate of pyranopterin. One of these enzymes is a radical SAM, a family of enzymes often associated with C—X bond-forming reactions (X = S, N).[8][7][6] This intermediate pyranopterin is then converted to the molybdopterin via the action of three further enzymes. In this conversion, the enedithiolate is formed, although the substituents on sulfur remain unknown. Sulfur is conveyed from cysteinyl persulfide in a manner reminiscent of the biosynthesis of iron-sulfur proteins. The monophosphate is adenylated (coupled to ADP) in a step that activates the cofactor toward binding Mo or W. These metals are imported as their oxyanions, molybdate, and tungstate.
In some enzymes, such as xanthine oxidase, the metal is bound to one molybdopterin, whereas, in other enzymes, e.g., DMSO reductase, the metal is bound to two molybdopterin cofactors.[9]
Models for the active sites of enzymes molybdopterin-containing enzymes are based on a class of ligands known as dithiolenes.[10]
Tungsten derivatives
editSome bacterial oxidoreductases use tungsten in a similar manner as molybdenum by using it in a tungsten-pterin complex, with molybdopterin. Thus, molybdopterin may complex with either molybdenum or tungsten. Tungsten-using enzymes typically reduce free carboxylic acids to aldehydes.[11]
The first tungsten-requiring enzyme to be discovered also requires selenium (though the precise form is unknown). In this case, the tungsten-selenium pair has been speculated to function analogously to the molybdenum-sulfur pairing of some molybdenum cofactor-requiring enzymes.[12] Although a tungsten-containing xanthine dehydrogenase from bacteria has been found to contain tungsten-molybdopterin and also non-protein-bound selenium (thus removing the possibility of selenium in selenocysteine or selenomethionine form), a tungsten-selenium molybdopterin complex has not been definitively described.[13]
Enzymes that use molybdopterin
editEnzymes that use molybdopterin as cofactor or prosthetic group are given below.[5] Molybdopterin is a:
- Cofactor of: xanthine oxidase, DMSO reductase, sulfite oxidase, nitrate reductase, ethylbenzene dehydrogenase, glyceraldehyde-3-phosphate ferredoxin oxidoreductase, respiratory arsenate reductase, carbon monoxide dehydrogenase, aldehyde oxidase.
- Prosthetic group of: formate dehydrogenase, purine hydroxylase, thiosulfate reductase.
See also
editReferences
edit- ^ "HPEUEJRPDGMIMY-UHFFFAOYSA-N". pubchem.ncbi.nlm.nih.gov. Retrieved 4 February 2019.
IUPAC Name [2-amino-4-oxo-6,7-bis(sulfanyl)-3,5,5~{a},8,9~{a},10-hexahydropyrano[3,2-g]pteridin-8-yl]methyl dihydrogen phosphate
- ^ "HPEUEJRPDGMIMY-UHFFFAOYSA-N". pubchem.ncbi.nlm.nih.gov. Retrieved 4 February 2019.
InChI InChI=1S/C10H14N5O6PS2/c11-10-14-7-4(8(16)15-10)12-3-6(24)5(23)2(21-9(3)13-7)1-20-22(17,18)19/h2-3,9,12,23-24H,1H2,(H2,17,18,19)(H4,11,13,14,15,16)
- ^ "[2-Amino-4-oxo-6,7-bis(sulfanyl)-3,5,5a,8,9a,10-hexahydropyrano[3,2-g]pteridin-8-yl]methyl dihydrogen phosphate". pubchem.ncbi.nlm.nih.gov. Retrieved 4 February 2019.
InChI=1S/C10H14N5O6PS2/c11-10-14-7-4(8(16)15-10)12-3-6(24)5(23)2(21-9(3)13-7)1-20-22(17,18)19/h2-3,9,12,23-24H,1H2,(H2,17,18,19)(H4,11,13,14,15,16)
- ^ "HPEUEJRPDGMIMY-UHFFFAOYSA-N". pubchem.ncbi.nlm.nih.gov. Retrieved 4 February 2019.
InChI Key HPEUEJRPDGMIMY-UHFFFAOYSA-N
- ^ a b Structure, synthesis, empirical formula for the di-sulfhydryl. Archived 2016-06-04 at the Wayback Machine Accessed Nov. 16, 2009.
- ^ a b Hover BM, Tonthat NK, Schumacher MA, Yokoyama K (May 2015). "Mechanism of pyranopterin ring formation in molybdenum cofactor biosynthesis". Proc Natl Acad Sci USA. 112 (20): 6347–52. Bibcode:2015PNAS..112.6347H. doi:10.1073/pnas.1500697112. PMC 4443348. PMID 25941396.
- ^ a b Hover BM, Loksztejn A, Ribeiro AA, Yokoyama K (April 2013). "Identification of a cyclic nucleotide as a cryptic intermediate in molybdenum cofactor biosynthesis". J Am Chem Soc. 135 (18): 7019–32. doi:10.1021/ja401781t. PMC 3777439. PMID 23627491.
- ^ Mendel, R. R.; Leimkuehler, S. (2015). "The biosynthesis of the molybdenum cofactors". J. Biol. Inorg. Chem. 20 (2): 337–347. doi:10.1007/s00775-014-1173-y. PMID 24980677. S2CID 2638550.
- ^ Schwarz, G. & Mendel, R. R. (2006). "Molybdenum cofactor biosynthesis and molybdenum enzymes". Annual Review of Plant Biology. 57: 623–647. doi:10.1146/annurev.arplant.57.032905.105437. PMID 16669776.
- ^ Kisker, C.; Schindelin, H.; Baas, D.; Rétey, J.; Meckenstock, R.U.; Kroneck, P.M.H. (1999). "A structural comparison of molybdenum cofactor-containing enzymes". FEMS Microbiol. Rev. 22 (5): 503–521. doi:10.1111/j.1574-6976.1998.tb00384.x. PMID 9990727.
- ^ Lassner, Erik (1999). Tungsten: Properties, Chemistry, Technology of the Element, Alloys and Chemical Compounds. Springer. pp. 409–411. ISBN 978-0-306-45053-2.
- ^ Stiefel, E. I. (1998). "Transition metal sulfur chemistry and its relevance to molybdenum and tungsten enzymes" (PDF). Pure Appl. Chem. 70 (4): 889–896. doi:10.1351/pac199870040889. S2CID 98647064.
- ^ Schräder T, Rienhöfer A, Andreesen JR (September 1999). "Selenium-containing xanthine dehydrogenase from Eubacterium barkeri". Eur. J. Biochem. 264 (3): 862–71. doi:10.1046/j.1432-1327.1999.00678.x. PMID 10491134.